2040
Improving sensitivity of infiltrative glioma detection by multi-parametric magnetic resonance imaging1In Vivo Imaging Facility, Department of Oncology, Luxembourg Institute of Health (LIH), Luxembourg, Luxembourg, 2NorLux Neuro-Oncology Laboratory, Department of Oncology, Luxembourg Institute of Health (LIH), Luxembourg, Luxembourg, 3KG Jebsen Brain Tumour Research Center, Department of Biomedicine, University of Bergen, Bergen, Norway, 4NorLux Neuro-Oncology, Department of Biomedicine, University of Bergen, Bergen, Norway
Synopsis
Glioblastoma is characterized by poor prognosis and limited treatment efficacy. One main contributing factor is the presence of a large population of infiltrated tumor cells that are difficult to visualize and treat with resective surgery and radiochemotherapy. In the present study, we aim at establishing techniques that combine various contrast mechanisms available in MRI and PET to improve the sensitivity of the detection of infiltrated tumour cells. Such techniques are likely to improve prognosis by early tumor detection, better delineation of the target for radiotherapy, and better assessment of the full extent of the tumor and its response to therapy.
Introduction
Glioblastoma (GBM), the most malignant form of brain tumors, is characterized by poor prognosis and limited treatment efficacy. One main contributing factor is the presence, at the time of diagnosis, of a large population of infiltrated tumor cells that are difficult to visualize and treat with resective surgery and radiochemotherapy1. This infiltrative compartment is thus often associated with the high recurrence after conventional treatments2. Imaging methods that can visualize the infiltrated tumor cells with high sensitivity are needed to improve prognosis by early tumor detection, better delineation of the target for radiotherapy, and better assessment of the full extent of the tumor and its response to therapy.
Our lab has established orthotopic patient-derived xenografts of GBM3, that recapitulate the clinical features of GBM and display phenotypes that can be angiogenic, infiltrative, or intermediate4. In previous studies, these models made it possible to illustrate how anti-angiogenic therapy of GBM can lead to a more infiltrative progression of the tumour, not easily detected by conventional imaging methods5. In the present study, we aim at establishing techniques that combine various contrast mechanisms available in MRI and PET to improve the sensitivity of the detection of infiltrated tumour cells.
Methods
We used preclinical models illustrative of the different GBM phenotypes: P13 (angiogenic), P3 (intermediate), T251 (invasive), and T476 (invasive). Magnetic Resonance Imaging (MRI) was performed on a 3T preclinical scanner and included sequences for relaxometry and diffusion. Functional maps of the longitudinal changes in T1, T2 and ADC are calculated and compared to values from non-tumour bearing animals. The scans were acquired weekly, using 5 mice per group.Results
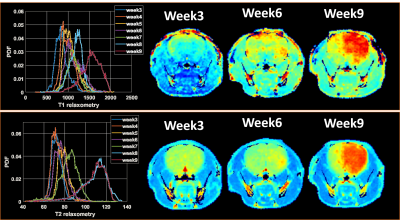
Each group is characterized by different growth rates and survival: 4 weeks for P13, 6 weeks for P3, 8 weeks for T251, and 9 weeks for T476. Early results suggest that T2 relaxometry may be the most sensitive method for the detection of the invasive phenotype (T251 & T476), being able to detect tumours up to two weeks before conventional T2w sequences. T1 relaxometry appeared to perform better for the angiogenic phenotype (P13).
Histogram analysis of tumors ROIs in relaxometry maps interestingly show a marked increase of the relaxometry values over time and a widening of the distribution (Figure 1).
Discussion
Preliminary results show the complementarity information provided by T1 and T2 relaxometry data in characterizing the different GBM phenotypes. Histogram analysis further suggests that functional maps displaying changes of these parameters over time (Fig. 1) and their comparison with normal tissue can further contribute to improve the sensitivity of the detection. Other contrast mechanisms, including diffusion and amide proton transfer, are also under investigation and may further contribute to the early detection of infiltrative tumors.
We will also present the results of an ongoing study to compare the sensitivity of multi-parametric MRI to that of amino acid FET-PET and the potential complementarity of these modalities.
Conclusions
The results call for a multimodal strategy to assess all the physiological changes that can result from the tumor growth. Different MRI contrast techniques provide complementary information, and a comparison of the changes over time and in comparison with healthy tissue has the potential to improve the sensitivity of detection of infiltrative tumor cells.Acknowledgements
References
1. Price SJ et al., Imaging biomarkers of brain tumour margin and tumour invasion, Br J Radiol, 2011
2. Akbari H et al., Imaging Surrogates of Infiltration Obtained Via Multiparametric Imaging Pattern Analysis Predict Subsequent Location of Recurrence of Glioblastoma, Neurosurgery, 2016
3. Wang et al., A reproducible brain tumour model established from human glioblastoma biopsies, BMC Cancer. 2009
4. Bougnaud S et al., Molecular crosstalk between tumour and brain parenchyma instructs histopathological features in glioblastoma, Oncotarget. 2016
5. Keunen O et al., Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma, Proc Natl Acad Sci U S A, 2011