2039
Variable flip angle RARE for High-Resolution Preclinical Brain and Spinal Cord Imaging1Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Variable flip angle RARE imaging has seen widespread utility in clinical brain and body imaging, but it has not been available for similar gains in preclinical MRI. This work demonstrates implementation and applications of vfaRARE in a the rat brain and spinal cord.
Introduction
Variable flip angle Rapid Acquisition with Refocused Echoes (RARE; also known as fast spin-echo: FSE) have seen considerable clinical utility since their first demonstration1. However, they have not made their way to preclinical systems for similar gains in image quality and efficiency. This work details the implementation of the variable flip angle RARE (vfaRARE) method on a preclinical MRI system and its application to the brain and spinal cord of rats.Methods
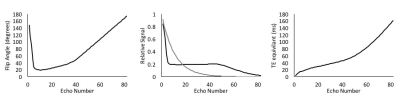
The iterative process described by Busse et al.1 was used to generate a flip angle schedule using the extended phase graph Matlab functions (http://med.stanford.edu/bmrgroup.html). T1 and T2 of the spinal cord at 9.4T of 1750 and 43 ms2,3, respectively, were simulated with an echo spacing of 4 ms and 80 echoes.
The calculated flip angle schedule was integrated into Paravision 6.0.1 (Bruker). In vivo rat brain imaging used a 9.4T Bruker Biospec with a 4-channel surface receive coil and a 9 cm diameter volume transmission coil. A 3D acquisition with a matrix of 160x152x72 and field of view of 28x26.5x12.5 mm3 (175 μm3 isotropic resolution) was used with spatial saturation bands above and below the brain along with frequency-selective fat suppression. The readout direction was A-P, and the excitation was a dorsal-ventral slab. An echo spacing of 4.6 ms was used with a 2ms SLR excitation pulse and 1ms slice-selective refocusing pulses. The first 6 echoes were discarded to allow for signal stabilization, and 76 echoes were acquired for each shot with the center of k-space occurring at the center of the echo train (linear encoding), yielding an equivalent echo time of 54ms. With a TR=4s, NEX=1, and full k-space sampling without acceleration, the scan time was 9:36min.
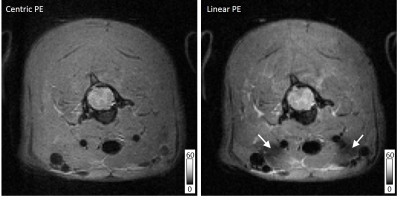
Imaging of the cervical spinal cord was also performed using a 38mm Litz coil (Doty Scientific) for transmission and reception. Parameters were identical to those above, except for the following: an isotropic acquisition with a field of view of 30x30x9.6mm, matrix of 128x128x32, RARE factor of 64, TR/TE: 2000/3.25 ms, NEX=2, and an acquisition time of 4:16 min. Both a centric and linear phase encoding strategy were evaluated using these parameters. Spinal cord imaging was respiratory triggered.
Results
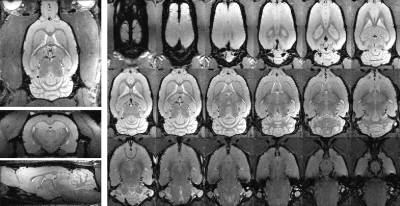
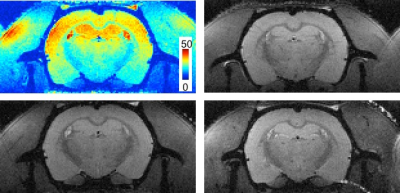
A representative in vivo 3D whole-brain dataset is shown in Fig 2. Isotropic high-resolution images (175 μm3) were obtained with minimal distortion or other artifacts. The signal intensity variability is caused by the receiver coil sensitivity profiles. A representative SNR map from the same dataset is shown in Fig. 3 (top left) with coronal images from 3 additional rats shown in the same figure. The average whole-brain SNR(N=4) at a coronal slice (Bregma=0mm) was 29.8(±2.9).
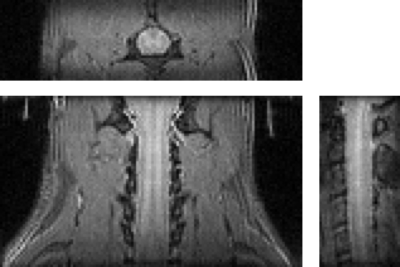
The vfaRARE sequence was also investigated in the cervical spinal cord first using an axial acquisition. At a lower resolution than that of the brain (300 μm3), the whole-cord SNR was slightly greater for the linear encoding(46.8) compared to centric encoding(42.7), and the CNR between white and gray matter was also slightly greater for the linear(4.91) versus centric(4.30) encoding. A region of signal loss was noted in the linear encoding images. In a sagittal 3D vfaRARE acquisition (also at 300 μm3 isotropic in 4:16min), the spinal cord anatomy is clearly visualized without ghosting or other artifacts.
Discussion
Variable flip angle fast spin-echo sequences have seen utility in many applications by multiple vendors, but this approach has not been previously available on preclinical systems. This approach offers higher efficiency compared to CPMG fast-spin echo sequences with reduced specific absorption rates (SAR). Although SAR is not typically a concern in preclinical studies, long spin-echo trains can lead to animal heating. Moreover, shorter relaxation times at high field necessitate shorter echo times and therefore longer scan times.
In the applications demonstrated here, images have high-resolution and are acquired in reasonable scan times that would be typically 3-4 times longer than their counterparts with uniform flip angles. Additional improvements may be made through partial k-space and parallel imaging accelerations to enable higher resolution or reduced scan times. For the spinal cord, additional methods to perform reduced field of view imaging would improve the resolution. The vfaRARE readout is also compatible with preparation modules such as inversion recovery, magnetization transfer, or motion-sensitization (i.e. diffusion). Most of these are implemented but were not tested.
The vfaRARE method has also shown utility in many other areas such as body, musculoskeletal, and peripheral nerve imaging. Applications to other anatomical regions in preclinical studies will be needed to realize its full potential as truly translational imaging tool.
Acknowledgements
This project was funded partially through the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin (MCW), the Craig H. Neilsen Foundation, and the US Department of Veterans Affairs Rehabilitation Research and Development Service. The sequences will be made available for download at: https://github.com/mdbudde/PreclinicalNeuroMRIReferences
1Busse et al; MRM 2006. 2van do Ven et al. Proc ISMRM. 2006. 3McCreary CR et al, Proc ISMRM 2006.Figures