2038
Combined MRI and Ultrasound Measurements to Assess the Impact of Systemic Chemotherapy on the Developing Brain and HeartLeigh Spencer Noakes1, Thomas Przybycien2, Amanda Forwell3, Yu-Qing Zhou1, Ellen van der Plas2, and Brian J. Nieman1,4
1The Mouse Imaging Centre, The Hospital for Sick Children, Toronto, ON, Canada, 2The Hospital for Sick Children, Toronto, ON, Canada, 3University of Waterloo, Waterloo, ON, Canada, 4Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Combined multiple-mouse ex vivo MRI and high-frequency cardiac ultrasound were used to assess the impact of common chemotherapy agents on the developing brain and heart. Of the eight agents considered, vincristine had the most widespread impact on the brain. Doxorubicin, methotrexate, and L-asparaginase were also found to impact brain and/or heart development.
Introduction
Acute lymphoblastic leukemia is the most common childhood cancer, with >3,000 new cases reported annually in the USA alone. Recent advances in medicine have improved survival rates to more than 90%1. However, survivors are often left with “late effects”, which include cognitive deficits and high risk of cardiac dysfunction, that result in a reduced quality of life2. The complex regimen of chemotherapy treatment—10 or more agents delivered over three years—is a considered a likely cause of late effects. Using a mouse model, we have sought to determine the individual impact of the most common chemotherapy agents on the brain and heart using a combination of ex vivo multiple mouse MRI and high frequency cardiac ultrasound. Our goal in this work was to systematically evaluate brain and cardiac outcomes after treatment with 8 essential ALL chemotherapy agents. Systematic quantification of the impact on brain and heart individually was the primary objective, but assessment of brain-heart relationships will also be explored.Methods
Infant CD-1 mice were treated intravenously with a single chemotherapy agent at postnatal day (P)17 and P19 and then allowed to age until early adulthood at P63. Agents tested were vincristine (VCR), doxorubicin (DXR), methotrexate (MTX), cytarabine (Ara-C), dexamethasone (DXS), L-asparaginase (L-ASE), and 6-mercaptopurine (6-MP). At P63 mice underwent in vivo cardiac ultrasound measurements and were perfusion fixed for high resolution (40 mm, isotropic) ex vivo MRI. MRI images were registered together nonlinearly through a series of iterative steps to produce an unbiased average. In the ex vivo MRI data, structurewise volumetric changes were computed by registering a segmented atlas with 159 structures to the unbiased average image. The volumes of each of the structures in the atlas were computed by summing over the structure volume, using the Jacobian determinant at each voxel to compute volumes from individual mice. Chemotherapy-treated mice were compared to vehicle-treated littermate controls. Ultrasound measurements focused on left ventricle (LV) structure and function, including measures of systolic and diastolic diameter, LV wall thickness, aorta diameter, E/A ratio, and the velocity-time integral for blood passing from the LV through the aorta. From these measurements, the cardiac output and left ventricle fractional shortening were calculated.Results
MRI measurements showed that, of the agents tested, VCR, DXR and MTX treatments resulted in the most severe impairments in brain development, shown in Figure 1 (a). VCR-treated mice having widespread volume deficits when compared to littermate controls. Both VCR and MTX had a significant impact on white matter volume, while DXR predominantly affected grey matter (although cerebellar white matter was also affected). Cardiac ultrasound revealed that MTX and L-ASE caused an increase in aorta diameter, as compared to control mice; while DXR caused in increase in E/A ratio (a marker of restrictive filling); and L-ASE also affected the left ventricle fractional shortening, this occurred more significantly in females. Results are shown in Figure 1 (b-e). Potential relationships between MRI and ultrasound outcome measures are also being explored.Conclusion
Combined use of in vivo ultrasound and high-resolution ex vivo MRI provide a method for high throughput assessment of the impact of chemotherapy on heart and brain development. Using this approach, we have identified systemic administration of VCR, DXR, and MTX and DXR, MTX, and L-ASE as impairing brain and heart development respectively.Acknowledgements
No acknowledgement found.References
1. Ries LAG, Melbert D, Krapcho M, Mariotto A, et al. SEER Cancer Statistics Review. 2007.
2. Van der Plas E, Schachar RJ, Hitzler J, et al. Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain Behav. 2017;7(2):e00621. doi:10.1002/brb3.621.
Figures
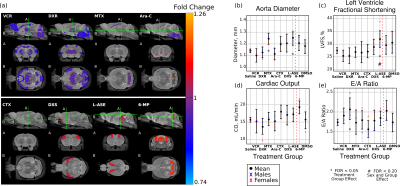
Figure 1. (a) Volume-wise maps showing the regional impact of each
chemotherapy agents. Areas in blue represent regions where neuroanatomical
volumes are smaller as a result of treatment, while areas in red represent
regions that are larger. VCR, DXR and MTX show the most wide spread impact on
the brain. (b-d) Cardiac ultrasound measurements of the aorta diameter, left
ventricle fractional shortening, cardiac output, and E/A ratio, respectively. Significant
changes are observed in the MTX-, DXR-, and L-ASE-treated mice.