2035
High Resolution Structural MRI of the of Eye: Initial Experience at Ultra High Field1Melbourne Brain Centre Imaging Unit, Department of Anatomy and Neuroscience, University of Melbourne, Parkville, Australia, 2Department of Optometry and Vision Sciences, University of Melbourne, Parkville, Australia
Synopsis
While optical eye imaging techniques are available for examining anterior and retinal structures, they are limited in making 3 dimensional assessments of the whole eyeball. MRI is the preferred modality in these areas but fine eye structures are difficult to resolve on clinical systems. Ultra high field magnets offer increased signal-to-noise, providing higher resolution, but there have been only a limited number of studies so far. We performed an initial study to assess achievable resolution, the anatomy visible on differing image weightings and MR parameter measurements, in eyes of healthy subjects on a 7 Tesla system.
Introduction
Optical eye imaging techniques (such as optical coherence tomography) are excellent, widely available methods for examining anterior and retinal structures. However, these have a limited role in 3D assessments of the whole eyeball and related orbital and retrobulbar structures. Magnetic resonance imaging is the preferred non-invasive modality for these regions but fine structures such as extraocular muscles, blood vessels and the optic nerve can be difficult to resolve on conventional clinical systems. Ultra high field magnets offer superior signal-to-noise, which may be ideal for providing the increased resolution needed to visualise these areas, but there have been a limited number of studies thus far[1,2,3]. We performed a preliminary assessment of achievable resolution, anatomy visible on differing image weightings and MR parameter measurements, in eyes of healthy subjects at ultra high field. This forms part of ongoing work seeking to optimise our imaging for future patient studies.Methods
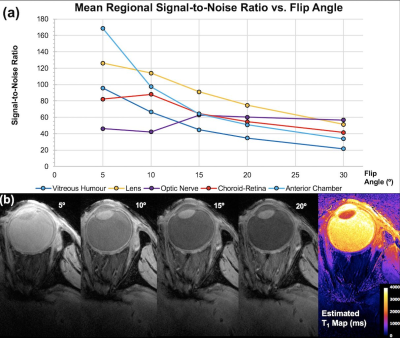
Subjects: All human imaging was conducted with Research Ethics Committee approval. Healthy volunteers (5M/2F, 29-47yrs) with normal acuity were imaged using a Siemens 7 Tesla research MRI scanner and dedicated 6-channel eye coil[3] (MRI.TOOLS GmbH, Berlin, Germany). Subjects performed a visual attention task in the system to maintain eye fixation. Select subjects underwent higher resolution scanning of one eye taped comfortably to reduce susceptibility and blink artefacts[2,4,5]. Imaging: Axial and sagittal 2D T2-w fast spin echo sequence (TEeff/TR/ETL/FA/NSA/TAcq: 11ms/5.4sec/18/170°/1/2mins) 0.26mm in-plane, 0.7mm slices. 3D gradient-echo (GRE) images were acquired one eye at a time (TE/TR/FA/GRAPPA/NSA/TAcq:10ms/4ms/12°/2/1/2or4mins) 0.2x0.2x0.4 or 0.9mm. One subject had 3D-GRE imaging at multiple flip angles (5-30°) and an additional TE. B1 maps were collected. Image processing: Images were N4 bias field corrected (Advanced Normalisation Tools) prior to anatomical viewing. Estimated T1 maps calculated from registered (NiftyReg, TIG, University College London UK) multiple FA 3D GRE datasets using JIM v5.0 (Xinapse Systems Ltd., West Bergholt UK).Results
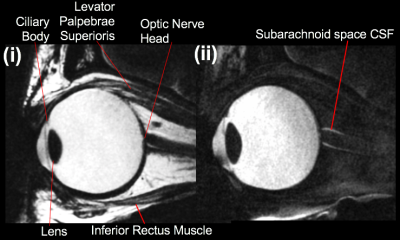
As seen in Fig. 1, the T2-w protocol produced detailed images with and without fat suppression. Fig. 2 shows the effect of varying flip angle on eye structure SNR in the 0.9mm 3D GRE images. These acquired images enabled voxel-by-voxel T1 estimates to be calculated. Shown in Fig. 3, imaging a taped eye, combined with effective fixation, allowed high-resolution T1-w images (voxel volume 0.016mm3) with low motion and susceptibility artefact and high SNR (mean whole-eye SNR = 37).Conclusion
This initial work demonstrates high resolution images of the eye on both T2 and T1-w sequences, with excellent anatomical detail including: the lens, ciliary body, optic nerve, ophthalmic vessels and arachnoid space CSF. For the GRE sequence in particular, we achieved a voxel volume of 0.016mm3 – lower than previous studies2,3 – which readily allowed volumetric structural segmentation. Further continued optimisation of these sequences may allow higher resolution, potentially increasing sensitivity to structural pathology in future patient studies.Acknowledgements
J.C. is a University of Melbourne McKenzie Fellow. This project and B.N. are supported by Melbourne Neuroscience Institute Seed Grant and Fellowship. S.K. is an Australian National Medical and Health Research Council Peter Doherty Fellow. Our 7T system is supported by the Australian National Imaging Facility (NIF). High-performance computing provided by the Multi-modal Australian ScienceS Imaging and Visualisation Environment (MASSIVE).References
1. Beenakker, J. W. M., et al. (2013) High‐resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed, 26(12), 1864–1869.
2. Richdale, K., et al. (2009). 7 Tesla MR imaging of the human eye in vivo. JMRI, 30(5), 924–932.
3. Graessl, A., et al. (2014). Ophthalmic magnetic resonance imaging at 7 T using a 6-channel transceiver radiofrequency coil array in healthy subjects and patients with intraocular masses. Investigative Radiology, 49(5), 260–270.
4. Bert, R. J., et al. (2006). High-resolution MR imaging of the human eye (2005). Academic Radiology, 13(3), 368–378.
5. Berkowitz, B. A., et al. (2001). Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: Eliminating blinking artifacts and demonstrating proof of concept. Mag Reson Med, 46(2), 412–416.
Figures