2034
In vivo MR detection and automated quantification of amyloid plaques in a preclinical model of Alzheimer's diseaseSteve J Sawiak1, Anne-Sophie Herard2, Mathieu D Santin3, Thierry Delzescaux2, and Marc Dhenain2
1Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 2MIRCen, CEA-CNRS, Fontenay aux Roses, France, 3ICM, Paris, France
Synopsis
Amyloid plaque load is a key index of disease burden in Alzheimer’s disease, but methods for its quantification are slow and operator dependent. Recent advances in the use of contrast agents allow the plaques to be visualized in vivo, but as yet no direct quantification methods are available. Here we present a new technique for automatic segmentation of amyloid plaques and to evaluate age-related or therapy related changes on a voxel-based basis with minimal user intervention. We report localized age-related changes of amyloid load across the whole brain of APP/PS1 mouse model of amyloidosis.
Objectives
β amyloid (Aβ) plaques deposition is one of the major neuropathological events associated to Alzheimer’s disease (AD). Amyloid deposition precedes and triggers a series of pathological events eventually leading to the onset of clinical AD [1]. Because of their very early occurrence, amyloid plaques are a major target for several disease modifying agents undergoing clinical development [2]. Magnetic resonance imaging (MRI) combined with intra-cerebro-ventricular administration of Gadolinium (Gd-DOTA) contrast agents can be used to detect individual amyloid plaques in transgenic live mice (ICV-Gd-staining protocol [3]). Once amyloid plaques have been detected on MRI, it becomes critical to be able to quantify the amyloid load in order to evaluate the impact of mechanisms or therapies that modulate the amyloid load. Our objective was to implement an automated protocol to segment amyloid plaques from MRI in order to facilitate the estimation of amyloid load during preclinical therapeutic evaluation.Methods
Experiments were conducted on 13 female APP/PS1 transgenic mice overexpressing amyloid precursor protein (APP) and/or presenilin 1 (PS1) mutations associated with familial AD. Detection of amyloid plaques was based on the administration of a gadolinium derivative contrast agent, gadoterate meglumine (Gd-DOTA, Dotarem®, Guerbet, France), to the animals as previously described [3, 4]. Briefly, 1 µl (0.5 mmol/mL) of Dotarem was injected into each hemisphere of the mice at a rate of 0.2 µl/min. For each mouse, in vivo MRI was performed twice at the age of 5.5 and 8.5 months on a 7T-spectrometer (Agilent, USA) using a high-resolution 3D-Gradient echo sequence (resolution: 29*29*117 µm3, field of view: 15*15*15mm3, matrix=512*512*128, TR=50ms, TE=25ms, flip angle=20°, number of averages=2, bandwidth=25kHz, acquisition time: 1h49min [3]). MR images were recorded starting at 60 minutes after administration of the Gd-DOTA contrast agent. During the MRI experiment the animals were anesthetized with a mixture of isoflurane (0.75 -1.5%) and carbogen (95% O2 - 5% CO2) and their breathing rate was monitored. Carbogen was used to reduce the signal coming from the circulating blood. Image processing was performed using SPM8 with the SPMMouse toolbox (http://spmmouse.org) [5] and BrainVisa (http://brainvisa.info/index_f.html). First, MR images were bias corrected, then rigid registrations were performed to achieve a common alignment of each subject with a C57Bl6 mouse templates. Amyloid plaques detected on MR images are hypointense and have a signal intensity that is similar to that of WM. We thus created prior maps with 20% level of WM within GM areas (Figure 1). The average image of the rigidly-aligned brains was segmented using a k-means algorithm [6] with 4 segments: background, GM-20%, WM-20%, and CSF-20%. So called segmented-WM maps represented the sum of two maps: real WM maps and amyloid plaques maps detecting amyloid within cortical regions. The amyloid plaque maps were extracted. The amyloid maps were output in rigid template space and DARTEL [7] was used to create non-linearly registered maps for each subject and common templates for the cohort of animals. Then the amyloid plaque maps were smoothed with an adaptive isotropic Gaussian kernel of 600µm. At each point in space the kernel excluded voxels that were marked as WM in the original, unmodified SPMMouse template to avoid smoothing between distinct structures. A general linear model was used to evaluate aging effects on amyloid load. Histological evaluation was performed at the end of the experiment to detect amyloid plaques from tissue sections.Results
Gd-staining allowed plaque detection by MRI (Figure 2a). Segmentation of MRI with SPM mouse was able to create probability amyloid plaque maps with segmented amyloid plaques (Figure 2b). Plaques from segmented MR images were registered to amyloid plaques detected by histology for validation and a good colocalization was seen between plaques detected on segmented-MRI and histological sections.The segmented maps were used to detect the regions with significant age-related increases of amyloid load and revealed amyloid deposition in deep cortical layers from most parts of the brain (Figure 3).Conclusion
Here, we show that amyloid plaques can be detected by in vivo MRI with a very high in-plane resolution (29µm) and segmented automatically. The protocol presented here can be used as a rapid and reliable method for quantification in anti-amyloid drug development trials covering the whole brain with minimal user intervention.Acknowledgements
Medicen (Pôle_de_compétitivité Île-de-France, TransAl_program), France-Alzheimer association, BPI.References
1. Hardy, J. Science, 2002. 297(5580):353-6; 2. Mangialasche, F. Lancet Neurol, 2010. 9(7): 702-16; 3. Petiet, A. Neurobiol Aging, 2012. 33(8): 1533-44; 4. Santin, M.D.; NeuroImage, 2013. 79: 288-294; 5. Sawiak, S.J. Magn Reson Imaging, 2013. 31(9): 1522-31; 6. MacKay, D.J.C. 2003, Cambridge: Cambridge University Press. Xii; 7. Ashburner, J., Neuroimage, 2007. 38(1): 95-113.Figures
C57Bl6 mouse WM, GM
and CSF templates (0%) and adapted 20% templates with 20% level of WM within GM
areas.
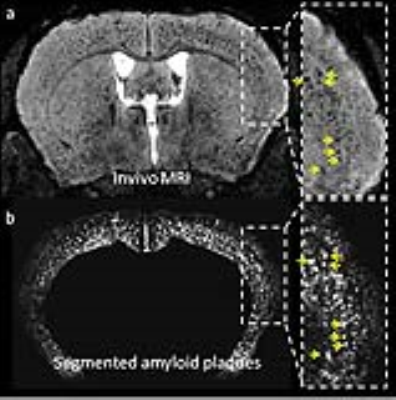
a. Detection of
amyloid plaques as hypointense spots in APP/PS1 mice. b. Probability amyloid
plaque maps obtained after segmentation of MRI with SPM mouse.
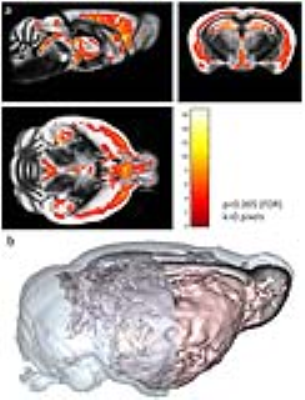
a. Voxel-based analysis showing significant age-related increases of amyloid load in APP/PS1 mice. b. 3D view of the
region with high age-related increases amyloid load.