2029
Noradrenaline shortage accelerates metabolic alterations in a transgenic model of Alzheimer´s disease1Biomedizinische NMR Forschungs GmbH, Max-Planck-Institut für biophysikalische Chemie, Göttingen, Germany, 2Abteilung Gene und Verhalten, Max-Planck-Institut für biophysikalische Chemie, Göttingen, Germany
Synopsis
Cerebral MRS of APP/PS1/Ear2(-/-) mice in vivo reveals significant alterations of several metabolites suggesting (i) an impaired cellular respiration compensated for by accelerated anaerobic glycolysis (i.e., elevated lactate), (ii) a loss of neurons (reduced N-acetylaspartate, glutamate, total creatine, and γ-aminobutyric acid) possibly compensated for by osmoregulators (elevated myo-inositol and taurine), (iii) an accumulation of paramagnetic iron (shortened water proton T2) possibly associated with inflammation, and (iv) subsequent gliosis (elevated myo-inositol). More specifically, a 60-75% reduction of noradrenaline is shown to accelerate the reduction of N-acetylaspartate and glutamate in the hippocampus as well as the T2-shortening in the frontal cortex.
Purpose
Noradrenaline (NA), also called norepinephrine, is a catecholamine that
acts as hormone and neurotransmitter. It is involved in general arousal, selective
attention, memory, and stress reactivity as well as in inflammation and neurodegeneration1.
The purpose of this work was to examine whether a shortage of NA influences the
pathology in a transgenic mouse model of Alzheimer´s disease.Methods
Animals. Thirteen aged (≥12 month-old) female C57BL/6N wild-type mice, 17 aged APP/PS1/Ear2(-/-) (4 male, 13 female) mice, 12 aged APP/PS1 mice (4 male, 8 female), and 4 aged female Ear2(-/-) mice were used. The animals were generated as described previously2,3.
MRS. At 9.4 T (Bruker Biospin MRI GmbH, Ettlingen, Germany), localized proton MRS (STEAM, TR/TE/TM = 6000/10/10 ms) was performed with the use of a birdcage resonator (inner diameter 70 mm) and a saddle-shaped quadrature surface coil (both Bruker Biospin MRI GmbH, Ettlingen, Germany) on anesthetized mice as previously described4. Volumes-of-interest were localized in the frontal cortex (1.8×1.8×1.0 mm3) or centered on the hippocampal formation (1.8×1.8×1.2 mm3), respectively. Water saturation was accomplished by means of three Gaussian-shaped CHESS radiofrequency pulses (90°-90°-180°) with a duration of 7.83 ms and a bandwidth of 350 Hz. Metabolite quantification involved spectral evaluation by LCModel5 and calibration with brain water concentration6, for which the unsuppressed water proton signal served as internal reference. The attenuation of the unsuppressed water signal was calibrated with T2 relaxation times of water protons in the volume-of-interest that were determined by multi-echo spin-echo MRI (TR/TE=2500/10–123 ms). T1 relaxation times were determined with the use of a spin-echo saturation recovery sequence and 7 TR values from 0.15 to 6 s. Metabolites with Cramer-Rao lower bounds above 20% were excluded from further analysis. Significant differences between two groups of data were determined by the Mann-Whitney´s U-test.
Results
MRS revealed significant alterations of several metabolite concentrations in the brain of APP/PS1/Ear2(-/-) mice in vivo (Figs. 1 and 2, Tables 1 and 2). These results are in line with previous MRS findings in human Alzheimer´s disease7. The MRS changes suggest an impaired cellular respiration compensated for by accelerated anaerobic glycolysis (i.e., elevated lactate), a loss of neurons (reduced N-acetylaspartate, glutamate, total creatine, and γ-aminobutyric acid) possibly compensated for by osmoregulators (elevated myo-inositol and taurine), an accumulation of paramagnetic iron (shortened water proton T2) possibly associated with inflammation8, and subsequent gliosis (elevated myo-inositol). More specifically, a 60-75% reduction of NA levels (APP/PS1 vs. APP/PS1/Ear2(-/-)) in projection areas, e.g., frontal cortex and hippocampus, is responsible for the reduction of N-acetylaspartate (9.5±1.9 vs. 7.4±0.9 mM, p<.01) and glutamate (11.9±1.7 vs. 9.4±1.4 mM, p<.01) in the hippocampus as well as for the water proton T2-shortening (39.0±1.1 vs. 37.8±0.7 ms, p<.05) in the frontal cortex (Table 3). These findings are in agreement with impaired spatial memory, disturbed synaptic plasticity, and increased astrogliosis as reported earlier1. The reduced glutamate concentration is in line with the assumption of positive feedback loops between NA and glutamate8.Discussion
The application of MRS to a transgenic mouse model of Alzheimer´s disease uncovered that chronic NA shortage enhances metabolic alterations indicating accelerated pathologic processes. Given that brain NA concentrations are associated with aging, dementia, Aβ plaque load, and the progression of Alzheimer´s disease1, it is foreseeable that MRS of the brain in laboratory animals as well as in humans plays an increasing role in translational biomedical research of Alzheimer´s disease.Conclusion
Shortage of NA in the brain accelerates the pathology in a transgenic model of Alzheimer´s disease.Acknowledgements
The authors wish to thank Prof. Dr. Gregor Eichele for helpful discussion.References
1. Kummer MP et al. J. Neurosci. 34, 8845-8854 (2014). 2. Jankowsky JL. Biomol. Eng. 17, 157-165 (2001). 3. Warnecke M et al. Genes Dev. 19, 614-625 (2005). 4. Watanabe T et al. Neuroimage, 133, 390-398 (2016). 5. Provencher SW. 1993. Magn. Reson. Med. 30, 672-679. 6. Duarte JMN et al. 2014. Neurobiol. Aging 35, 1660-1668. 7. Shonk TK et al. Radiology, 195, 65-72 (1995). 8. Watanabe T et al. Mag. Reson. Med. Sci. 15, 11-25 (2016). 9. Berridge CW & Waterhouse BD. Brain Res. Rev. 42, 33–84 (2003).Figures
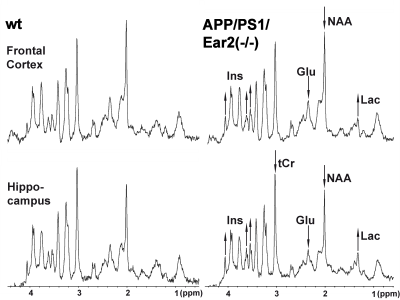
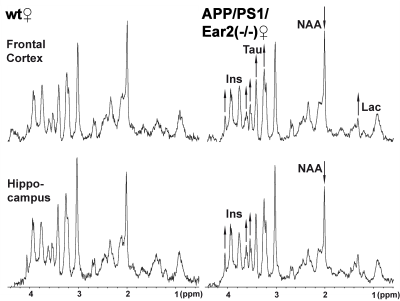
Table 1 Concentration (mM) and concentration ratio of major cerebral metabolites as well as T1 and T2 relaxation times of water protons in APP/PS1/Ear2(-/-) and wild-type control mice.
GABA = γ-aminobutyric acid, GPC = glycerophosphocholine, NAAG =N-acetylaspartylglutamate, PCh = phosphocholine, Tau = taurine, tCr = creatine + phosphocreatine. *, **, *** = p < .05, p < .01, p < .001, respectively, vs. wild type in Mann-Whitney´s U-test. For other abbreviations see Fig. 1.
Table 2 Concentration (mM) and concentration ratio of major cerebral metabolites as well as T1 and T2 relaxation times of water protons in female APP/PS1/Ear2(-/-) and wild type control mice.
*, ** = p < .05, p < .01 vs. wild type in Mann-Whitney´s U-test. For abbreviations see Fig. 1 and Table 1.
Table 3 Concentration (mM) and concentration ratio of major cerebral metabolites as well as T1 and T2 relaxation times of water protons in APP/PS1/Ear2(-/-) compared to APP/PS1 mice.
*, ** = p < .05, p < .01 vs. APP/PS1 in Mann-Whitney´s U-test. For abbreviations see Fig. 1 and Table 1.