2027
Iron Deposition in Alzheimer's Dementia Hippocampus is Associated with Increased R2* Values1Electrical Engineering, Brigham Young University, Provo, UT, United States, 2Physiology and Developmental Biology, Brigham Young University, Provo, UT, United States
Synopsis
We describe the utilization of UTE-3D Cones to create T2* maps of iron deposition in the hippocampus of an Alzheimer’s dementia subject, but not in a corticobasal degeneration subject. These results are consistent with histopathological studies involving post-mortem human brain tissue. UTE-3D Cones could be a promising imaging protocol for AD diagnostic imaging.
INTRODUCTION
Alzheimer’s disease (AD) – a progressive, neurodegenerative, and incurable disorder – is the most common form of dementia diagnosed in people over age 65. It looms as a major threat to public health in the first half of the 21st Century. Dementia doubles in frequency every five years after age 60, afflicting 1% of those aged 60-64 but rising to 30-40% of those 85 years and older1. Growing evidence demonstrates that oxidative stress is an important factor contributing to the initiation and progression of AD2-6. Iron is an essential trace mineral required for proper neuronal function but if iron becomes free in the neuron or surrounding environment, its redox transitions between Fe(II) and Fe(III) generate radicals. Additionally, Fe(III) binds to hyper-phosphorylated tau (HP-tau) and induces its aggregation into tangles. HP-tau aggregation is reversible in vitro by chemically reducing Fe(III) to Fe(II), indicating the possibility that similar treatments may be possible to eliminate tau tangles in vivo7. Our own preliminary immunohistochemistry studies have shown that in the medial temporal lobe, iron co-localizes with amyloid beta (Aβ) plaques, and/or with HP-tau, depending on the anatomical region, severity of AD diagnosis, and age of the patient at death. These findings are particularly important since iron can be visualized with Magnetic Resonance Imaging (MRI). Indeed, our preliminary T2* imaging studies with post-mortem human brain specimens and concomitant histopathological analysis demonstrate that iron co-localization with AD pathological proteins is dependent on anatomy, disease severity, and age. Therefore, iron imaging has noteworthy promise as a potential imaging biomarker for AD.METHODS
We acquired whole-brain images from a 76-year-old male diagnosed with corticobasal degeneration (CBD), and a 78-year-old female diagnosed with mild to moderate Alzheimer’s dementia (AD) was recruited and scanned in accordance with IRB procedures. Images were acquired on a Siemens Tim Trio system using a 32-channel head coil. A total of three different imaging protocols were used: A T1-weighted MPRAGE, a-T2 weighted SPACE for anatomical structures, and a series of UTE-3D Cones with echo times of 0.25, 0.5, 0.75, 1, 2, and 5 ms to generate a UTE-T2* map. Parameters for the various scans are shown in Figure 1. Raw data for the 3D-Cones sequence was collected and reconstructed offline using MATLAB. R2* maps were produced with MATLAB’s curve fitting tool using a monoexponential model. A mask was drawn around the brain to reduce the time required to calculate fits.RESULTS
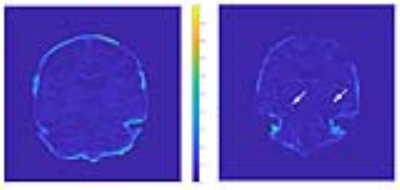
Results show an elevated R2* value (i.e. shortened T2*) in the dorsal hippocampus (corresponding to CA1-CA4 subfields) of the hippocampus in the AD subject, but not in the CBD subject, suggesting the presence of iron deposits (Figure 2) consistent with our previous post-mortem/histopathology matched studies. Compared to surrounding normal tissue, R2* values in the dorsal hippocampus increased from 0.03 to 0.1 indicating a much more rapid signal decay. Various areas of the cerebral cortex in both the CBD and AD subjects also exhibit increased R2* values, but not to the extent displayed in the dorsal hippocampus of the AD subject.DISCUSSION
In our preliminary histopathology studies, we found that the shortened T2* values corresponded with the presence of Aβ plaques, and/or with HP-tau. T2* values were markedly lower when iron and microglial activation was associated with these proteins. MRI is a non-invasive, non-radioactive imaging modality with high resolution and anatomical specificity. Given the importance of functional neuroanatomy in AD, its contrasting pathological process with other neurodegenerative diseases, and the association of iron co-localization with the pathological proteins involved with the disease, T2* mapping has significant potential as a diagnostic tool.Acknowledgements
We would like to acknoledge funding from the National Institutes of Health 2 R01 EB002524-10.References
1. Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta psychiatr scand. 1987;76:465-79.
2. Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2013;2013:316523. Epub 2013/08/29. doi: 10.1155/2013/316523. PubMed PMID: 23983897; PMCID: 3745981.
3. Mondragon-Rodriguez S, Perry G, Zhu X, Moreira PI, Acevedo-Aquino MC, Williams S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: implications for Alzheimer's disease. Oxidative medicine and cellular longevity. 2013;2013:940603. Epub 2013/08/13. doi: 10.1155/2013/940603. PubMed PMID: 23936615; PMCID: 3723250.
4. Friedman A, Arosio P, Finazzi D, Koziorowski D, Galazka-Friedman J. Ferritin as an important player in neurodegeneration. Parkinsonism Relat Disord. 2011;17(6):423-30. Epub 2011/05/10. doi: 10.1016/j.parkreldis.2011.03.016. PubMed PMID: 21550835.
5. Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345(1-2):91-104. Epub 2010/08/24. doi: 10.1007/s11010-010-0563-x. PubMed PMID: 20730621.
6. Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33(4):457-63. Epub 2002/08/06. PubMed PMID: 12160928.
7. Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer's disease. J Neurochem. 2002;82(5):1137-47. Epub 2002/10/03. PubMed PMID: 12358761.
Figures