2020
APPswe/PS1dE9 mice with cortical amyloid pathology show a reduced NAA/Cr ratio without apparent brain atrophy: A MRS and MRI study1Institute for Experimental Surgery, Rostock University Medical Center, Rostock, Germany, 2Rostock University Medical Center, Rostock, Germany, 3Core Facility Multimodal Small Animal imaging, Rostock University Medical Center, Rostock University Medical Center, Rostock, Germany, 4Institute of Diagnostic Radiology & Neuroradiology, University Medicine Greifswald, Gerifswald, Germany, 5Department of Nuclear Medicine, Rostock University Medical Center, Rostock, Germany, 6German Center for Neurodegenerative Diseases (DZNE), Rostock, Germany
Synopsis
Amyloid-ß deposition is one of the hallmarks of Alzheimer’s disease (AD) that starts to progress decades before the onset of cognitive impairment. With the rise of the new diagnostic criteria of AD that considers the neuropathological changes as the main aspects for explaining the extent of the disease regardless the cognitive status of the patient & further highlighted the importance of finding reliable in-vivo biological markers to identify those in the preclinical stage of AD. Through the use of the transgenic mice models, particularly APPswe/PS1dE9 we could study the different pathomechanics contributing to the development of AD. So, in this study, we assumed an approach combining morphometry based on high-resolution MRI as a
Introduction
Amyloid-ß deposition is one of the hallmarks of Alzheimer’s disease (AD) that starts to progress decades before the onset of cognitive impairment. With rise of the new diagnostic criteria of AD that considers the neuro-pathological changes as the main aspects for explaining the extent of the disease regardless the cognitive status of the patient & further highlighted the importance of finding reliable in-vivo biological markers to identify those in the preclinical stage of AD (6). Transgenic mice models offers a good opportunity for studying the different pathomechanics contributing to the development of AD, particularly APPswe/PS1dE9 which is one of the most widely used models, in which amyloid plaques deposition is initiated at the age of 4-6 months followed by other morphological alterations (6). So, we assumed an approach combining morphometry based on high resolution MRI & proton magnetic resonance spectroscopy together with the histopathological assessments of neuron & amyloid plaques load in APPswe/PS1dE9 mouse, to assess the interaction between histological & neuroimaging markers of neuronal loss , neuronal function & brain atrophy (2).Methods
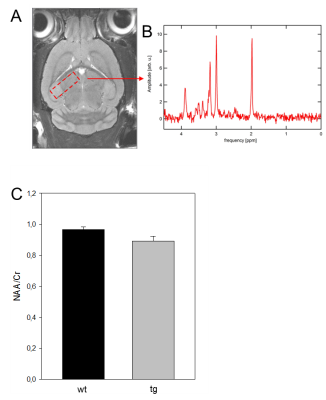
We used for this study double transgenic female APPswe/PS1dE9 (tg) mice on the genetic background C57BL/6J & C3H/HeJ (n=10) at the age of 12 months. For the controls, wild type (wt) age-matched littermates (n=9) were used. All mice were anesthetized with 1-3% isoflurane in 100% oxygen then scanned in a 7T small animal MRI (Brucker). The imaging protocol included morphological high resolution T2-weighted (RARE sequence) followed MRS using stimulation echo acquisition method (STEAM) with outer volume suppression sequence. All the mice are sacrificed after experimental procedures then brain tissue was harvested for histopathological analysis. The voxel-based morphometric analysis of the T2-weighted RARE sequence were carried out using SPM 8 implemented within MATLAB (1, 4) & MRS data were analyzed using jMRU software package to compute metabolites ratios as NAA/Cr ratio.Results
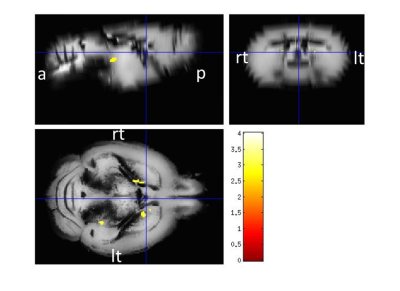
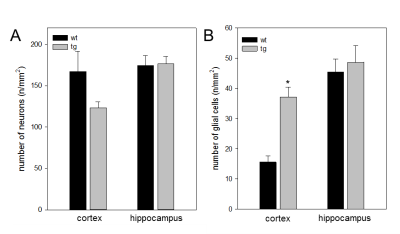
Regarding the morphometry, few scattered clusters of reduced grey matter volume could be detected in caudate, hippocampus & cerebellum in tg mice, however, the effects didn’t survive a p-value of p < 0.05 using false discovery rate. On the other hand, the MRS revealed a difference of 10% of NAA/Cr ratio in wt when compared to age-matched tg mice (3, 5). Immunohistochemically analyses showed that the tg mice revealed an up to 27% decrease in the number of cortical neuronal cells along with raised cortical gliosis when compared to wt mice. However, there was no appreciable difference detected in the hippocampus regions between the tg & wt mice.Conclusion
APPswe/PS1dE9 mice, as a model of AD showed an increased Aß plaques, loss of neurons & an impairment of NAA/Cr ratio (3) , which wasn’t accompanied with significant brain atrophy & subsequently this implies caution when projecting these experiments form mice models to humans.Keywords
APPswe/PS1 dE9 mice, neuronal loss, Aß-plaques, NAA/Cr ratio, brain atrophyAcknowledgements
No acknowledgement found.References
1- Ashburner, J., Friston, K., 1997. Multimodal image coregistration and partitioning–a unified framework. NeuroImage 6, 209–217.
2- Biedermann, S.V., Fuss, J., Steinle, J., Auer, M.K., Dormann, C., Falfán-Melgoza, C., Ende, G., Gass, P., Weber-Fahr, W., 2016. The hippocampus and exercise: histological correlates of MR-detected volume changes. Brain Struct. Funct. 221, 1353–1363.
3- Chen, S.Q., Cai, Q., Shen, Y.Y., Wang, P.J., Teng, G.J., Zhang, W., Zang, F.C., 2012. Age- related changes in brain metabolites and cognitive function in APP/PS1 transgenic mice. Behav. Brain Res. 235, 1–6.
4- Lau, J.C., Lerch, J.P., Sled, J.G., Henkelman, R.M., Evans, A.C., Bedell, B.J., 2008. Longitudinal neuroanatomical changes determined by deformation-based morphometry in a mouse model of Alzheimer's disease. NeuroImage 42, 19–27.
5- Mlynárik, V., Cacquevel, M., Sun-Reimer, L., Janssens, S., Cudalbu, C., Lei, H., Schneider, B.L., Aebischer, P., Gruetter, R., 2012. Proton and phosphorus magnetic resonance spectroscopy of a mouse model of Alzheimer's disease. J. Alzheimers Dis. 31 (Suppl.3), S87–S99.
6- Teipel, S.J., Buchert, R., Thome, J., Hampel, H., Pahnke, J., 2011. Development of Alzheimer-disease neuroimaging-biomarkers using mouse models with amyloid-precursor protein-transgene expression. Prog. Neurobiol. 95, 547–556.
Figures