2017
Functional and structural deficits in a novel transgenic rat model of Alzheimer’s Disease.1Bio-Imaging Lab, University of Antwerp, Wilrijk, Antwerp, Belgium, 2Department of Radiology, NYU Langone Medical Center, Center for Biomedical Imaging, New York, NY, United States, 3Spinal Cord Injury and Tissue Regeneration Center Salzburg; Institute of Experimental Neuroregeneration; Paracelsus Medical University, Salzburg, Austria, 4Wolfson Molecular Imaging Centre, University of Manchester, Manchester, United Kingdom
Synopsis
As improving our understanding of the underlying mechanisms of Alzheimer’s Disease (AD) pathology is of utmost importance, the development and characterization of innovative animal models is essential in AD-related research. Here, we further characterized a novel transgenic rat model of AD, the TgF344-AD rat, which manifests progressive AD pathology, much akin to human AD. Functional and structural deficits along the disease progression were assessed using resting state functional MRI (rsfMRI) and diffusion tensor imaging, respectively.
Introduction
The detection of early AD-related pathology is of utmost importance to improve our understanding of the role of soluble Aβ, Aβ plaques and neurofibrillary tangles (NFT) in the progression of AD. Therefore, the development and characterization of new animal models is pivotal in AD research as they enable the identification of early pathological processes, which are often not accessible in patients, and subsequent target discovery and evaluation. Although transgenic mouse models have been proven highly valuable in elucidating several pathological mechanisms involved in AD, they do not develop NFT unless additional human tau transgenes are included, cf. triple transgenic AD mouse model. Recently, a novel transgenic rat model was developed, the TgF344-AD rat model, which manifests progressive AD pathology similar to human AD. As such, this model presents progressive cerebral amyloidosis, tauopathy and neuronal loss, associated with age-dependent cognitive decline. As early as 6 months of age, this model shows a striking overabundance of soluble Aβ$$$_{1-40}$$$, that is, before the onset of appreciable plaque formation$$$^{1}$$$. Here, we examined AD-related pathology in this new rat model by using a combination of complementary in vivo MRI techniques. We hypothesized that the early pathological processes in AD would lead to aberrant functional connectivity (FC) measures, and that this would coincide with minor (early-stage) and major (late-stage) structural changes. More specifically, we studied (1) synaptic deficits using resting state functional MRI (rsfMRI), and (2) white and grey matter integrity based on diffusion tensor imaging (DTI).Methods
A total of 23 female TgF344-AD rats (N$$$_{WT}$$$=12, N$$$_{TG}$$$=11) were used in a longitudinal study (6, 10, 12, 16 and 18 months). Imaging procedures (7T Bruker Pharmascan, Ettlingen, Germany) were conducted on spontaneously breathing rats under isoflurane anaesthesia (induction: 5%; maintenance: 2%). Specific for the rsfMRI acquisition, animals were anaesthetized using a mixture of medetomidine (0.05mg/kg s.c. bolus; 0.01mg/kg/h s.c. infusion) and a low dose of isoflurane (0.4%). Each imaging session consisted of a rsfMRI scan (2D GE-EPI; TR/TE 2000/29.19 ms; 20 axial slices; 300 repetitions; spatial resolution (0.234 x 0.234 x 0.8)mm³), a DTI scan (2D DW-SE-EPI; TR/TE 7500/25.97ms; δ 4ms; Δ 12ms; b-factor 800 s/mm²; 60 diffusion directions; 20 slices; spatial resolution (0.234 x 0.234 x 0.8)mm³) and a T2-weighted 3D anatomical scan (RARE, TR/TE 3185/44ms; RARE factor 8; spatial resolution (0.11 x 0.25 x 0.20) mm³). Physiological parameters were continuously monitored and kept stable throughout the procedures. All data was spatially normalized to a study-specific 3D template to perform voxel-based analyses (VBA) in SPM12. For the rsfMRI data, an independent component analysis (ICA) was performed using a pre-set of 20 components. Additionally, region of interest (ROI) based analyses of FC and diffusion metrics were performed using linear mixed models in JMP Pro 13.
Results & Discussion
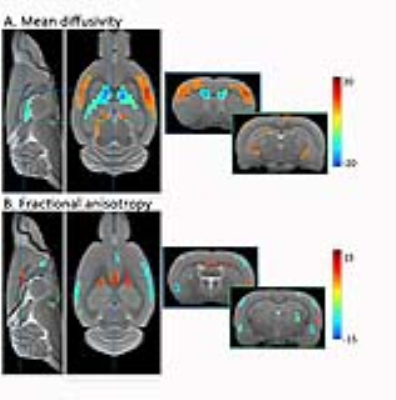
The outcome of a between-component FC analysis, using anatomically relevant ICA components as ROIs, is shown in Fig. 1. A strong genotype effect was present throughout the brain, with significantly reduced FC in TG animals compared to WT animals affecting regions characteristically involved in AD such as the hippocampus (HC), cingulate (Cg) and retrosplenial (RS) cortices. This decreased functional coherence in TG animals was further confirmed with a within-component analysis and seed-based analysis (Fig. 2). Next, a VBA of the DTI parameter maps revealed widespread changes of the structural integrity of the brain (Fig. 3). As such, increased MD was found in the sensory cortex of TG animals, whereas decreased values were present in the ventricular area. Furthermore, TG animals had reduced FA in the sensory cortex and several white matter structures, including the external capsule, whereas increased FA was found in the corpus callosum of TG rats. A more in-depth ROI-based examination of functional and structural parameters in the Cg, RS and HC regions indicated that although altered FC could be observed already at an early stage of the disease, with the largest difference at 10 months, DTI parameters changed mainly at later stages.Conclusion
Already at early stages, TgF344-AD rats displayed a strong and widespread attenuation of the brain’s functional architecture. This effect preceded structural alterations of the brain, which were only observed at later stages of the disease. The pre-existing FC differences, as seen in this study, could point towards a possible developmental effect in this model, whereas the structural changes seemed to follow disease progression more closely. Further ex vivo analyses will help in explaining the observed in vivo MRI results.Acknowledgements
This research was supported by the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement number 278850 (INMiND) and by the Fund for Scientific Research Flanders (FWO) (grant agreements G067515N and G057615N).References
1. Cohen, R.M., et al., A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci, 2013. 33(15): p. 6245-56.Figures