2016
Higher temporal lobe curvature in early Alzheimer's indicative of subsequent cognitive decline1Cleveland Clinic, Las Vegas, NV, United States, 2University of Colorado Boulder, Boulder, CO, United States
Synopsis
We selected ADNI patients with an initial diagnosis of mild cognitive impairment (MCI) due to early Alzheimer’s disease, a positive amyloid PET scan within 4 years, and comparable cognitive test scores during their initial visit. Patients were grouped according to their diagnosed outcome within 4 years of the initial visit, specifically, MCI subsequently diagnosed with dementia and stable MCI. We found that curvature within the temporal lobe was greater among patients subsequently diagnosed with dementia. Established measurements of atrophy, including hippocampal volume and temporal lobe thickness, did not differ between these groups.
Background
Mild cognitive impairment (MCI) due to early Alzheimer’s disease (AD) is an important population in AD research and treatment. Accurate diagnosis is crucial for clinical trials targeting the AD pathology underlying impairment. β-Amyloid tracers for positron emission tomography (PET) identify plaque buildup that is a hallmark of the AD pathology1. When combined with assessment tools such as the Alzheimer’s Disease Assessment Scale (ADAS) and the Clinical Dementia Rating Scale (CDR), a positive amyloid PET scan can help clinicians more accurately diagnose cognitive impairment as likely due to Alzheimer’s disease. Advancements in early diagnosis will aid research on disease progression in AD. Meta-analysis has shown that the population of early-AD patients tracked were newly diagnosed with dementia at approximately a 10% annual conversion rate, and 75% eventually developed dementia2. In cases where a patient is monitored from early diagnosis of mild impairment through to dementia, it may be possible to identify measures that have predictive value for dementia onset. Furthermore, these cases can be compared with cases of early-AD MCI patients who do not develop dementia during subsequent monitoring. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). ADNI participants are repeatedly tested with MRI, PET, and cognitive tests in an effort to track AD progression. We selected a subset of ADNI patients with at least 4 years of testing, an initial diagnosis of mild cognitive impairment (MCI) due to suspected early Alzheimer’s disease, and a positive amyloid PET scan within 4 years. Patients were grouped according to their diagnosed outcome within 4 years of the initial visit, specifically, MCI subsequently diagnosed with dementia (MCI-ADD) and stable MCI (MCI-MCI). We investigated morphological differences between groups in the initial visit T1 scans. The MCI-MCI and MCI-ADD groups selected for analysis are demographically and cognitively similar at the time of T1 acquisition, and only begin to diverge in annual follow-up testing. Group differences in the initial visit T1 analysis might indicate biomarkers for dementia onset risk in early Alzheimer’s disease.Methods
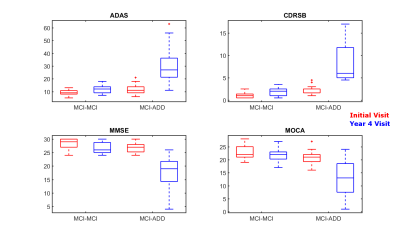
All 19 MCI-ADD and 19 MCI-MCI ADNI patients were β-Amyloid positive within 4 years of the initial visit, indicating a higher likelihood of Alzheimer’s disease pathology. All patients had a CDR of 0.5 at the initial visit, indicating mild impairment in the absence of dementia. MCI-ADD differed from the MCI-MCI at the year 4 visit: CDRSB > 4.5, along with significantly worse performance on ADAS, MMSE, and MOCA (Figure 2). Group demographics were comparable: MCI-ADD mean age 69 (SD 6.4) and years of education 15.7 (SD 2.4) with 11 females; MCI-MCI mean age 72 (SD 7.3) and education 15.4 (SD 2.7) with 9 females. 38 MPRAGE images were collected from the ADNI archive. All images were acquired during the initial visit of right-handed MCI patients. Images were processed entirely in Freesurfer version 6.03, using all steps of the cortical reconstruction process. In each hemisphere, we extracted volume measurements for 8 subcortical regions, along with volume, thickness, and curvature measurements for 34 cortical regions.Results
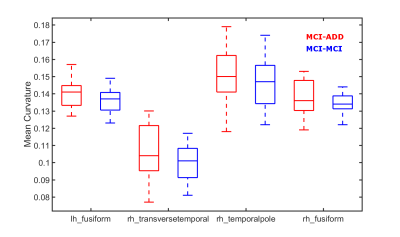
Statistical analysis of each Freesurfer ROI was performed using FSL’s Permutation Analysis of Linear Models (PALM)4. A separate analysis with 10,000 permutations was performed for each measurement (subcortical volume as well as cortical volume, thickness, and curvature). Uncorrected p values < 0.01 were chosen for mean curvature within the left hemisphere fusiform gyrus as well as the right hemisphere temporal-pole and tansverse-temporal gyri. Uncorrected p < 0.05 was chosen for the right hemisphere fusiform gyrus mean curvature. For these regions, we see higher curvature for MCI-ADD (Figure 1). We did not find any significant or marginally significant FWER-corrected or uncorrected differences in volume or thickness.
Discussion
Established measurements of atrophy, including hippocampal volume and temporal lobe thickness, did not differ for MCI patients who experienced subsequent cognitive decline and a dementia diagnosis. This suggests that atrophy is more correlated with current, rather than subsequent impairment, as cognitive test scores were comparable between groups at the time of T1 acquisition. We find that curvature within the temporal lobe is greater among patients diagnosed with dementia within 4 years of the MRI. Curvature can be conceptualized as the inverse radius of a sphere osculating the cortex surface5. Curvature is an indirect measure of cortical folding. Brain curvature has been observed to decline with age5, therefore, one possible interpretation of our findings is that the disease course of AD might involve a change in brain morphology that counteracts age-related smoothing of the cortex. Perhaps atrophy or disease severity is related to this observation.
Acknowledgements
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5P20GM109025.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative(ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson &Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; MesoScale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education,and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
Joshi, A.D., et al., Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer's disease and cognitively normal subjects. J Nucl Med, 2012. 53(3): 378-384
Bruscoli, M., Lovestone, S. Is MCI really just early dementia? A systematic review of conversion studies. International Psychogeriatrics, 2004. 16(2): 129–140
Dale, A.M., Fischl, B., Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 1999. 9: 179-194
Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage, 2014. 92: 381-397
Pienaar, R., Fischl, B., Caviness, V., Makris, N., Grant, P.E. A methodology for analyzing curvature in the developing brain from preterm to adult. Int J Imaging Syst Technol, 2008 Jun 1. 18(1): 42–68
Figures