2013
Understanding the Role of Gender in Progression and Severity of Alzheimer ’s Disease: A 1H-[13C]-NMR investigation1NMR Microimaging and Spectroscopy, CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India
Synopsis
The epidemiological data suggested more prevalence of AD in females than males. To understand the severity of AD in females, we have performed behavioral and neurometabolic analysis in female and male 3xTg-AD mice. Though, the learning and memory are impaired in both male and female AD mice, there is no neurometabolic impairment in female 3xTg-AD mice. In contrary, neurometabolism was severely compromised in male AD mice. The data from the current study suggest more severe AD in males as compared to females till their reproductive age.
INTRODUCTION
Alzheimer’s
disease (AD) is the most common neurodegenerative disorder associated with
gradual deterioration in memory and cognitive function1. Age and
gender are considered as major factors to influence the progression of
AD, and the number of women suffering from AD is
always higher than that of males in respective age groups from 60 years to 90. It may be attributed to the higher longevity of women at
higher age groups, but at the age of 60 to 80 difference in mortality of males
and females is not significant to account for the differences in the disease severity2. The 3xTg-AD mouse is an advanced model of AD which exhibit both Aβ plaques and neurofibrillary tangles3. The perturbation in neurometabolite homeostasis and regional glucose hypometabolism has been reported in the AD condition4,5. The objective of the current study is to assess the severity of AD in males and females by monitoring behavioral phenotype and neurometabolism using 3xTg-AD mice.MATERIALS AND METHODS
RESULTS AND DISCUSSIONS
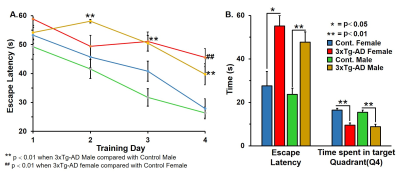
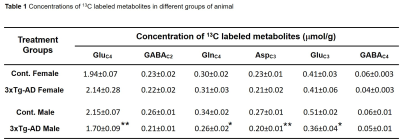
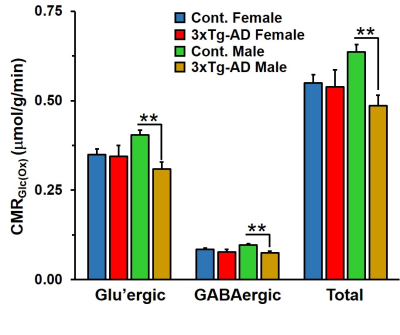
Learning of 3xTg-AD males and females was compromised when compared with the respective controls (Figure 1A). The memory assessment carried out on 7th day shows that escape latency of 3xTg-AD males (47.7±4.7 s vs 23.7±2.8 s, p=0.008) and females (55.1±4.8 s vs 27.5±6.6 s, p=0.013) was almost doubled as comapred with the respective control. Moreover, the retention of memory analysis suggested that time spent in quadrant 4 by male (8.8±1.1 s vs 15.4±1.0 s, p=0.001) and female (9.5±0.9 s vs 16.4±0.8 s, p=0.002) 3xTg-AD mice is significantly less than controls (Figure 1B). These results suggests learning and memory impairment in 3xTg-AD. The level of glutamate was decreased (Cont 12.9±0.3 μmol/g; 3xTg-AD 11.7±0.3 μmol/g; p=0.008), and choline was increased (Cont 0.05±0.01 μmol/g, 3xTg-AD 0.09±0.02 μmol/g, p=0.029) in male 3xTg-AD mice. Moreover, the concentrations of 13C lebeled GluC4 (p=0.001), GlnC4 (p=0.020), AspC3 (p=0.003) and GluC3 (p=0.012) were decreased significantly in male 3xTg-AD mice (Figure 2, Table 1) when compared with controls. There was no significant change in the level of neurometabolites and 13C labeling in female 3xTg-AD mice. The reduction in concentration of 13C labeled amino acids resulted into decreased metabolic activity of glutamatergic (Cont 0.40±0.01 μmol/g/min; 3xTg-AD 0.31±0.02 μmol/g/min; p=0.002) and GABAergic neurons (Cont 0.10±0.004 μmol/g/min; 3xTg-AD 0.07±0.004 μmol/g/min; p=0.004) in male 3xTg-AD mice when compared with controls (Figure 3). The neurometabolism was unpertubed in transgenic female mice. Although, there was a loss in learning and memory in case of female 3xTg-AD mice which is important behavioral phenotype in AD condition, there was no perturbation in neurometabolic activity. In contrast, 3xTg-AD male mice exhibited stronger behavioral phenotype and show pertubation in neurometabolite homeostasis and imapiment in neurometabolic activity.The data presented in the study provide an experimental evidence for increased severity of AD condition in 3xTg-AD male mice, and existence of some neuroprotective mechanism in female during their reproductive age. The severity of AD beyond the reproductive age needs further investigations.Acknowledgements
The study was supported by funding from CSIR-CCMB.References
1. Selkoe DJ (1989) Amyloid beta protein precursor and the pathogenesis of Alzheimer's disease. Cell 58:611-612.
2. Lloret et al. (2008) Gender and age-dependent differences in the mitochondrial apoptogenic pathway in Alzheimer’s disease. Free RadicBiol Med 44, 2019-2025.
3. Oddo et al. (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Ab and synaptic dysfunction.Neuron 39: 409-421
4. Tiwari V and Patel AB (2012) Impaired glutamatergic and GABAergic function at early age in AβPPswe-PS1dE9 mice: implications for Alzheimer's disease. J Alzheimers’s Dis 28:765-69.
5. Dubois A et al. (2010) Detection by voxel-wise statistical analysis of significant changes in regional cerebral glucose uptake in an APP/PS1 transgenic mouse model of Alzheimer's disease. Neuroimage 51:586-98.
6. Vorhees CV1, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc.1(2):848-58.
7. Epstein AA et al (2013) Combinatorial assessments of brain tissue metabolomics and histopathology in rodent models of human immunodeficiency virus infection. J Neuroimmune Pharmacol. 1224-38
8. Patel AB et al (2005) The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo proc. Nat Acad Sci USA 5588–5593
9. Tiwari V, Ambadipudi S, Patel AB. (2013) Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab 33:1523-31
Figures