2010
APOE ε4 Allele Effect on White Matter Perfusion and Diffusion in Cognitively Normal and MCI Groups1Radiology, Wake Forest School of Medicine, Winston-Salem, NC, United States, 2Gerontology and Geriatric Medicine, Wake Forest School of Medicine, Winston-Salem, NC, United States
Synopsis
Hypo-perfusion was observed among APOE ε4 carriers in both white and gray matter from the previous study in cognitively normal and mild cognitive impairment groups. Diffusion tensor imaging metrics in the white matter was further examined in the hypo-perfusion region and compared with perfusion metrics. Multiple statistical trends match with the observations from the perfusion metrics, which may suggest evidence of that a perfusion abnormality among APOE ε4 carriers may precedes the disruption of white matter integrity in the group.
Introduction
Hypo-perfusion in white matter (WM) is commonly observed in Alzheimer’s disease (AD) and an early SPECT study showed that significantly lower WM cerebral blood flow (CBF) in the parietotemporo-occipital area was observed in AD subjects compared to age-matched controls1. WM injury is an early feature of AD and several animal experiments demonstrate that cerebral hypo-perfusion induces the loss of oligodendrocytes and WM damage2. In our earlier study3, arterial spin labeling (ASL) images were analyzed in cognitively normal and mild cognitive impairment (MCI) groups, and lower CBF and shortened ATT were observed among APOE ε4 carriers in both white and gray matter. In this study, we examined the WM characteristics obtained with diffusion tensor imaging (DTI) in the hypo-perfusion region. Although the WM metrics did not significantly differ by the APOE genotype or cognitive status, multiple statistical trends match with the observations from the perfusion metrics, which may shed a light on the relationship between perfusion and diffusion in the group of APOE ε4 carriers.Methods
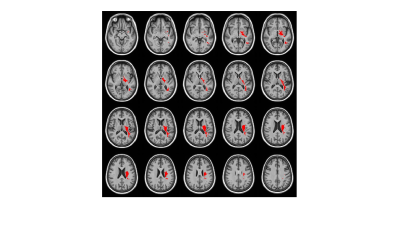
87 subjects (62 cognitively normal (CN) (23% ε4+) and 25 MCI (48% ε4+); 23M/64F; age: 63.9±7.7) underwent baseline brain MRI exams as part of several on-going studies related to within the Wake Forest AD Center, including multiple inversion time pseudo-continuous ASL to estimate voxel-wise whole brain CBF and ATT in white and gray matters using a partial volume correction4 and DTI (30 directions with b=1000) to estimate diffusion parameters including ones from a crossing fiber model. All ASL images were co-registered to T1 weighted structural images and normalized into a study specific template. Age, Sex, and BMI were adjusted and a 2-way ANOVA was performed for each voxel. A minimum cluster size of 450 voxels (3.6cm3) was determined with p<0.05 and α=0.05. A single ROI (size of 10.7cm3), which is located mainly in left sub-lobar region and extending over medical temporal and parietal lobes (see Figure 1), showed the significant hypo-perfusion region in the APOE ε4 carrier group. The hypo-perfusion cluster in the study specific template was transformed into each subject’s DTI space to compute mean values of DTI metrics. Age, Sex, and BMI were adjusted on the mean DTI metrics across subjects and a 2-way ANOVA was performed for group comparison.Results
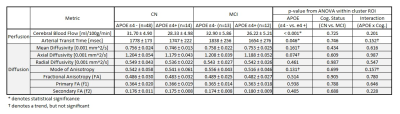
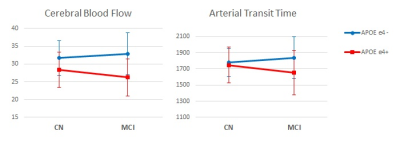
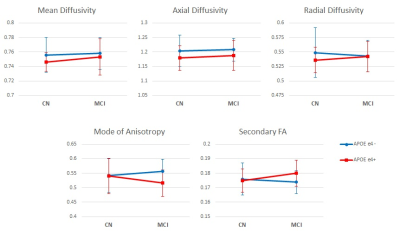
APOE ε4 carriers showed reduced CBF in a white matter cluster and the group without ε4 Allele showed a trend of compensatory increased blood flow in MCI while that with ε4 Allele had impaired blood flow as shown in Table I and Figure 2. In the region, diffusivity parameters, particularly mean and axial diffusivity, showed a trend of lower diffusivity in APOE ε4 carriers (upper plots in Figure 3): p=0.161 for mean diffusivity and p=0.074 for axial diffusivity. The mode of diffusivity reflecting the shape of diffusion tensor from linear (1) to planar (-1) represented a similar trend as CBF and the secondary FA showed the opposite pattern (lower plots in Figure 3).Discussion
Lower CBF and shortened ATT may represent possible vascular impairment, such as vessel narrowing or stiffness, in the location among APOE ε4 carriers and the affected perfusion may alter diffusion characteristics.
Lower diffusivity in the left parietal and medical temporal regions may relate to impaired blood flow and, therefore, lower blood volumes. To further investigate the relationship between diffusivity and structural volume, structural morphometry in the affected white matter may provide additional analytic measures for volume adjusted diffusivity. And structural morphometry of hippocampus, in addition, is of interest in that it may provide an indication that the hypo-perfusion and diffusion metric differences may precede hippocampal atrophy.
The hypo-perfusion pattern, a trend of compensatory increased blood flow in the group without ε4 Allele and MCI but impaired blood flow for those with ε4 Allele and MCI, was found in the measure of mode of diffusivity, which represents a transition from more linear to more planar shape of the diffusion tensor in white matter. It may reflect a disorganization of the crossing white matter tracts but it is still unclear that the FA measured in the secondary crossing fiber represents the opposite pattern. More precise WM Tract based normalization would allow to segment the effected WM region into single and crossing fiber regions and the separate analysis in the regions may provide better explanation of this relationship.
Conclusion
In this small pilot study sample, trends of reduced diffusivity and mode of anisotropy and elevated secondary FA were observed among APOE ε4 carriers in the hypo-perfusion region in white matter but did not differ by cognitive status. These data may suggest evidence of that a perfusion abnormality among APOE ε4 carriers may precedes the disruption of white matter integrity in the group.Acknowledgements
Wake Forest Alzheimer's Disease Core Center supported by NIH (P30AG049638)References
1. Bonte FJ, Ross ED, Chehabi HH, Devous Sr MD. SPECT study of regional cerebral blood flow in Alzheimer disease. Journal of Computer Assisted Tomography. 1986;10(4):579-83.
2. Farkas E, Donka G, de Vos RAI, Mihaly A, Bari F, Luiten PGM. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathologica. 2004;108(1):57-64.
3. Youngkyoo Jung, Megan E. Johnston, Jeongchul Kim, Christopher T. Whitlow, Timothy M. Hughes, Laura D. Baker, Suzanne Craft. APOE ε4 Allele Effect on White and Gray Matter Perfusion in Cognitively Normal and MCI Groups. Alzheimer's & Dementia, Vol. 12, Issue 7, P374–P375.
4. Youngkyoo Jung, Megan E Johnston, Christopher T Whitlow. Estimation of Cerebral Blood Flow and Arterial Transit Time Using Partial Volume Corrected Multi-TI Arterial Spin Labeling Imaging. In: Proceedings of 23th Annual Meeting of ISMRM, Honolulu, Hawaii, 2017. p 1881.
Figures