2007
Sex Differences in Behavior, Brain Structure and Functional Connectivity in the APOE Epsilon 4 Knock-In Rat Model of Alzheimer's Disease: Are Females the Stronger Sex?1Psychology, Northeastern University, Boston, MA, United States, 2Brain Imaging Center, McGill University, Montreal, QC, Canada
Synopsis
APOE genotypes are a major focus for Alzheimer’s disease (AD) research following the localization of ApoE on neurofibrillary tangles and amyloids of senile plaques AD patients’ brain. The risk of developing AD increases with the frequency of the ε4 allele, with women outnumbering men. In this study we utilized multiple imaging modalities and behavioral assays to identify sex-specific anatomical biomarkers in a novel rat APOE-ε4 knock-in model. ε4+ males show greater variation in neural structure and function in terms of the proportion of brain areas affected; these results are reflected in sex-driven differences in behavior mirroring hippocampal function.
Purpose
Several studies have noted sex-specific differences in brain morphology, neural function, and cognitive performance between human carriers and non-carriers of the APOE ε4 allele. These studies align with pre-clinical data from transgenic animals showing that neurobiological processes associated with the ε4 allele differently interact with sex to affect behavior and alter the structure of various regions of the brain. The purpose of this study was to characterize the anatomical, microstructural, and functional circuit differences that exist between male and female rats expressing the human APOE-ε4 allele.Methods
ROI volume assessment. T1- and T2-weighted high-resolution anatomical images were collected using the RARE pulse sequence. For T1-weighted images following parameters were used 35 slice of 0.7 mm thickness; field of view [FOV] 3 cm; 256 × 256; repetition time [TR] 988 msec; echo time [TE] 9.45 msec; NEX 6; 8 min49 sec acquisition time. T2-weighted images were collected with following parameters, 35 slice of 0.7 mm thickness; field of view [FOV] 3 cm; 256 × 256; repetition time [TR] 3900 msec; effective echo time [TE] 48 msec; NEX 3; 6 min 14 sec acquisition time. In the volumetric analysis, each brain region was segmented, and the volume values were extracted for all 171 ROIs, calculated by multiplying unit volume of voxel in mm3 by the number of voxels using in-house MATLAB script.
Diffusion Weighted Imaging. DWI was acquired with a spin-echo echo-planar-imaging (EPI) pulse sequence having the following parameters: TR/TE = 500/20 ms, eight EPI segments, and 10 non-collinear gradient directions. Geometrical parameters were: 48 coronal slices, each 0.313 mm thick (brain volume) and with in-plane resolution of 0.313 × 0.313 mm2 (matrix size 96×96; FOV 30 mm2). Image analysis included DWI analysis of the DW-3D-EPI images to produce the maps of fractional anisotropy (FA) and radial diffusivity (RD). For statistical comparisons between rats, each brain volume was registered with the 3D rat brain atlas allowing voxel and region based statistics. Functional connectivity. Resting-state fMRI scan were collected using spin-echo triple-shot EPI sequence (imaging parameters: matrix size= 96x96x20, TR/TE=3000/15 msec, voxel size=0.312x0.312x0.12mm, slice thickness= 1mm). Functional data were assessed for motion spikes, and large spikes were identified and removed in time-course signals, followed by slice timing correction from interleaved slice acquisition order. Head motion correction (six motion parameters) was carried out using the first volume as a reference image. Voxel time series data were averaged in each node based on the residual images using the nuisance regression procedure. Pearson’s Rs across all pairs of nodes were computed for each subject among all three groups to assess the interregional temporal correlations. Group-level analysis was performed assess the functional connectivity in controls and ε4+ groups.
Results
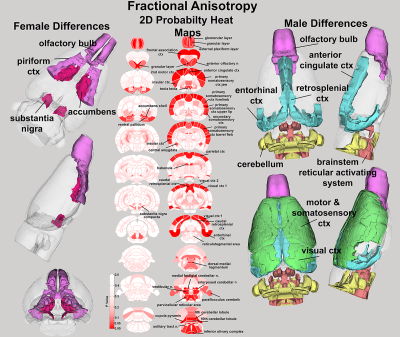
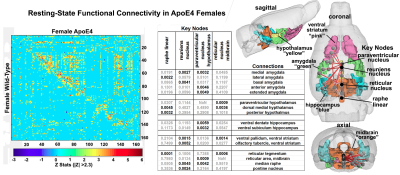
Behavioral analysis revealed that only male ε4+ animals have impaired stimulus recognition and object memory, while the results from Barnes maze analyses suggests that the ε4 allele impairs spatial memory and navigation equally across both sexes (Figure 1). Fractional anisotropy data reveal greater diffusivity between WT and ε4+ rats in males, but not females (Figure 2); the male WT/ε4+ difference was driven primarily by cortical regions (i.e. frontal association cortex, somatosensory cortices and entorhinal cortex). Furthermore, female ε4+ rats show different patterns of functional connectivity compared to the WT controls (Figure 3). In males, the collection of areas showing altered connectivity existed within four separate networks including the primary motor cortex, the dorsal raphe nucleus, the retrosplenial cortex, and the periaqueductal gray.Discussion and Conclusions
Compared to controls, ε4+ rats showed evidence of altered function and structure in various brain regions across all imaging modalities between male and females. In most cases, alterations were only present in males with very few instances of microstructural shifts specific to females. Generally, male ε4+ rats showed extensive increases in radial diffusivity across the cerebellar region that, in some cases, also showed decreased fractional anisotropy. Of particular note, the deep cerebellar nuclei as well as various hindbrain regions, including the locus coeruleus, showed altered diffusivity values. Taken together, these data show that the anatomical, functional and behavioral differences recorded in e4+ males are consistent with reports that indicate that interconnected hippocampal/parahippocampal circuits are involved in the regulation of object memory and recognition. Furthermore, the results point at a clear differentiation with regards to sex in the context of preclinical AD modeling, offering further support for sex/gender differentiation in neuroscience and subsequently, drug discovery/treatment.Acknowledgements
This work was funded by a Horizon Discovery grant to C.F.F.References
1. Madularu, D., Kulkarni, P., Ferris, C.F. & Brake, W.G. Changes in brain volume in response to estradiol levels, amphetamine sensitization and haloperidol treatment in awake female rats. Brain research 1618, 100-110 (2015).
2. Kenkel, W.M., et al. Functional magnetic resonance imaging in awake transgenic fragile X rats: evidence of dysregulation in reward processing in the mesolimbic/habenular neural circuit. Translational psychiatry 6, e763 (2016).
3. Dumais, K.M., Kulkarni, P.P., Ferris, C.F. and Veenema, A.H., 2017. Sex differences in neural activation following different routes of oxytocin administration in awake adult rats. Psychoneuroendocrinology, 81, pp.52-62.