2006
Regional brain iron accumulation in an Alzheimer’s mouse model fed lipophilic iron1Neurosurgery, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 2Radiology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States
Synopsis
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized pathologically by amyloid beta (Aβ) deposition, microgliosis, and iron dyshomeostasis. The goal of this work was to observe how brain iron levels temporally influence Aβ plaque formation, plaque iron concentration, and microgliosis. Humanized APPNL-G-F knock-in and control mice were fed either lipophilic iron compound 3,5,5-trimethylhexanoyl ferrocene (TMHF), normal, or iron deficient diets for twelve months. Increased brain iron was observed in the olfactory, frontal and hippocampal regions and was associated with increased plaque-iron loading and microglial iron inclusions.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized pathologically by amyloid beta (Aβ) deposition, microgliosis, and iron dyshomeostasis. Increased labile iron due to homeostatic dysregulation is believed to facilitate amyloidogenesis1. Free iron is incorporated into aggregating amyloid peptides during Aβ plaque formation and increases the potential for oxidative stress surrounding plaques. The goal of this work was to observe how brain iron levels temporally influence Aβ plaque formation, plaque iron concentration, and microgliosis.Methods
Humanized APPNL-G-F knock-in2 and wild-type C57BL6 mice were fed either lipophilic iron compound 3,5,5-trimethylhexanoyl ferrocene (TMHF)3,4 (0.11%, 200ppm Fe), normal iron (200ppm Fe), or iron deficient (trace, 2-6ppm Fe) diets for 3, 6, and twelve-months. An MRI was performed on each animal at 3 month increments; 3D-RARE T2, multi-echo T2, and multi-echo 3D GRE scans were obtained. The brain and liver were extracted and processed for histological, metal, and protein analysis. Brain homogenate and liver aliquots were measured for metal content and TMHF presence with ICP-MS and HPLC, respectively. Sagittal tissue sections were processed for Perl’s and laser ablation ICP-MS to histologically measure and localize metal content. Anti-Aβ42, anti-Aβ40, IBA1, GFAP, and L-Ferritin immunohistochemical stains were performed. MRI data was analyzed within SPM using the DARTEL and SMP-Mouse toolboxes. Glial, oxidative stress, and inflammation markers were regionally measured to determine the effect of dietary iron overload and the regional relationship to brain MRI relaxation parameters.Results
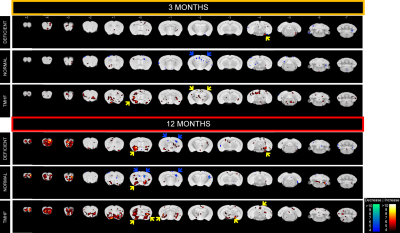
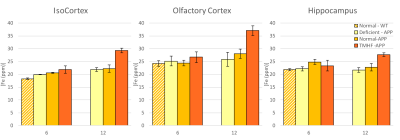
Longitudinal comparison of R2 between baseline to three and twelve months are displayed in Figures 1a and 1b. Animals fed the TMHF diet at three and twelve months had increased relaxation rate (R2) in the anterior olfactory nucleus, nucleus accumbens, caudate putamen, substantia innominata, globus pallidus, cingulate cortex, piriform cortex, lateral dorsal thalamic nucleus, internal and external capsule, midbrain, primary visual cortex, cerebellum, and pons. The TMHF diet elevated brain iron by 22% and the iron deficient diet decreased brain iron 21% relative to the control diet. Increasing brain iron accelerated plaque formation, decreased Aβ40 staining, and increased senile morphology of the amyloid plaques. Increased brain iron was associated with increased plaque-iron loading and microglial iron inclusions. TMHF decreased IBA1+ microglia branch length while increasing roundness which is indicative of microglial activation. Laser ablation iron concentration maps of control and APPNL-GF brains at six and twelve months illustrate progressive regional iron load with different iron diets (Fig. 2). Twelve-month TMHF brain tissue contains higher levels of iron within the olfactory bulb, hippocampus, iso-cortex (Fig. 3), midbrain colliculi, thalamic nuclei, cerebellum, fornix, and the splenium of the corpus callosum (not shown). More concentrated iron speckling appears in twelve-month APPNL-GF mouse fed TMHF relative to the six-month animal. Speckling appeared within the thalamus, basal ganglia, CA1, subiculum, and frontal cortex, indicative of further abnormal iron deposition within those areas.Discussion
This body of work suggests that increasing mouse brain iron with TMHF potentiates a more human-like Alzheimer’s disease phenotype with iron integration into Aβ plaques and associated microgliosis. The data demonstrates that TMHF elevates brain iron in a region-specific manner, increases the rate of early amyloid plaque deposition, and intensifies iron load within plaques. The MRI, laser ablation, and Perl’s stain data concurrently demonstrate that the hippocampus and frontal cortex of TMHF fed animals contain Aβ plaques with focal iron deposits.Conclusion
The data support elevated iron infiltration and earlier Aβ plaque formation within the gray matter of TMHF fed animals, consistent with work that illustrates AD brain tissue has increased infiltration of iron into frontal gray matter areas5.Acknowledgements
No acknowledgement found.References
1. Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer's disease: Two sides of the same coin. Neurobiol Dis. Sep 2015;81:49-65.
2. Saito T, Matsuba Y, Mihira N, et al. Single App knock-in mouse models of Alzheimer's disease. Nat Neurosci. May 2014;17(5):661-663.
3. Malecki EA, Cable EE, Isom HC, Connor JR. The lipophilic iron compound TMH-ferrocene [(3,5,5-trimethylhexanoyl)ferrocene] increases iron concentrations, neuronal L-ferritin, and heme oxygenase in brains of BALB/c mice. Biol Trace Elem Res. Apr 2002;86(1):73-84.
4. Nielsen P, Heinelt S, Dullmann J. Chronic feeding of carbonyl-iron and TMH-ferrocene in rats. Comparison of two iron-overload models with different iron absorption. Comp Biochem Physiol C. Oct 1993;106(2):429-436.
5. Hare DJ, Raven EP, Roberts BR, et al. Laser ablation-inductively coupled plasma-mass spectrometry imaging of white and gray matter iron distribution in Alzheimer's disease frontal cortex. Neuroimage. Aug 15 2016;137:124-131.
Figures