2005
MRI and PET alterations in Alzheimer’s disease and cognitive normal HFEH63D polymorphism carriers1Neurosurgery, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 2Radiology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States
Synopsis
Anatomical MRI and PET data from the genetic cohort of the ADNI database was analyzed for MRI volumetric, FDG-PET, and AV-45 differences between HFEH63D polymorphism and HFEWT carriers. A decrease in the amount of AV-45 amyloid binding was observed in the AD HFEH63D carriers as well as an increase in FDG metabolism and a decrease in regional brain volume. HFEH63D appears to be preservative in AD with respect to PET imaging biomarkers, but there was a negative interaction in the VBM analysis. This reinforces the hypothesis that HFEH63D has a preservative effect in AD.
Introduction
HFE polymorphisms have been implicated in the development and progression of neurodegenerative diseases, a phenomena which is generally attributed to iron dyshomeostasis. AD-HFEH63D carriers would be expected to have more significant signs of pathology than AD-HFEWT subjects under this hypothesis, but recent studies have shown the reverse trend with HFE knock-out model mice having less AD related brain protein transcripts 1 and AD-HFEH63D carriers having lower brain atrophy rates2. We further tested the hypothesis that HFEH63D has an opposite, preservative effect in AD compared to its effect in cognitively normal subjects, using VBM analysis and PET imaging.Methods
Data from 432 subjects (305 AD/MCI) from the ADNI cohort were used in this study, selected based on the presence of genetic sequencing data. Anatomical T1, FDG-PET, and AV-45 PET images were downloaded and pre-processed using AFNI. T1 images were additionally processed using the DARTEL VBM toolbox in SPM8 to investigate volumetric differences between groups. Subjects were separated into 4 groups based on cognitive status and HFEH63D genotype, and statistical analyses were carried out using SPM8.Results
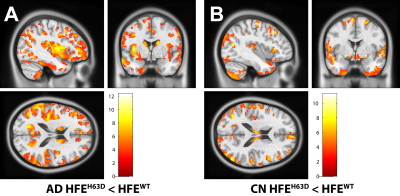
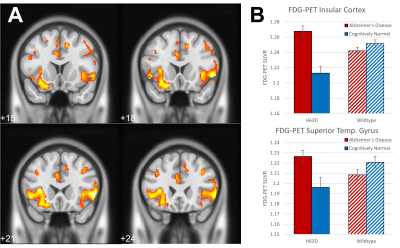
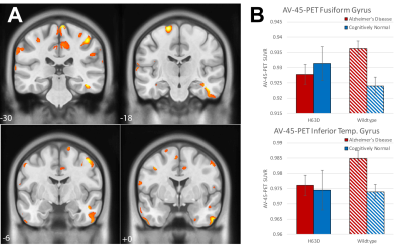
Volumetric analysis showed areas of greater volume in the middle frontal gyri in AD-HFEH63D carriers, but otherwise smaller insula, cingulate, inferior and orbital frontal gyri, caudate, and a large extent of the temporal lobe. In contrast, the CN-HFEH63D carriers exhibited larger lingual and orbitofrontal gyri, but less insular cortex volume. HFEH63D in AD subjects resulted in higher FDG signal in the anterior insula, cingulate gyrus, middle frontal gyri, and the putamen, with no corresponding loss of FDG signal at p<0.001. Cognitively normal HFEH63D subjects only differed in higher signal in the lateral frontal gyrus and most anterior part of the insula. AD-HFEH63D carriers had reduced AV-45 signal in middle and inferior frontal gyri, and the postcentral and superior temporal gyri. AD-HFEH63D carriers also had a highly significant increase in AV-45 signal in the brainstem below the level of the pons. There was no consistent differences in AV-45 signal between the cognitively normal groups.Discussion
While VBM analysis did not indicate any protective role, PET imaging analysis supported the hypothesis that HFEH63D was protective in AD subjects while being disadvantageous in cognitively normal subjects. Other studies have shown that HFE mutations alter levels of some mRNA transcripts known to influence AD risk or progression1, which could modify pathology in AD-HFEH63D subjects. We hypothesize that the HFEH63D polymorphism could be promoting gliosis at a cost of myelination, improving AD-HFEH63D subjects’ ability to clear Aβ. The resulting white matter loss could present as atrophy, even as cell bodies and metabolic activity are preserved. It is beyond the capability of in vivo neuroimaging to discern the source or cause of atrophy, and would require postmortem or animal studies to investigate the source of amyloid and volumetric differences.Conclusion
The HFEH63D polymorphism effect on the brain appears opposite in cognitively normal and AD subjects. AD-HFEH63D subjects appear protected compared to AD-HFEWT, approaching measures observed in cognitively normal subjects, while CN-HFEH63D subjects more resemble AD pathology compared to CN-HFEWT subjects. HFEH63D does appear to be preservative in AD subjects for metabolic activity and amyloid deposition, but not measures of atrophy.Acknowledgements
No acknowledgement found.References
1. Johnstone DM, Graham RM, Trinder D, et al. Changes in brain transcripts related to Alzheimer's disease in a model of HFE hemochromatosis are not consistent with increased Alzheimer's disease risk. J Alzheimers Dis. 2012;30(4):791-803.
2. Wang ZX, Wan Y, Tan L, et al. Genetic Association of HLA Gene Variants with MRI Brain Structure in Alzheimer's Disease. Mol Neurobiol. Jul 2017;54(5):3195-3204.
Figures