1999
Influence of Different Anesthesia Protocols on Cerebrovascular Reactivity and Cerebral Blood Flow measured by Pseudo-continuous Arterial Spin Labeling1Department of Radiology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Anesthesia protocols in animal studies greatly influence cerebral hemodynamics, so it is critical to devise standardized protocols in order to provide reproducible and comparable Cerebral Blood Flow (CBF) and Cerebral Vascular Reactivity (CVR) in different mouse strains or models. We compared strain-dependent sensitivity towards different anesthesia protocols for vascular reactivity experiments (high-dose isoflurane, medetomidine, low-induction dose isoflurane and high-dose isoflurane in intubated and mechanically ventilated mice), using pseudo-Continuous ASL (pCASL) and discuss the relative performance of these protocols.
Introduction
Cerebrovascular reactivity (CVR) and baseline cerebral blood flow (CBF) are considered to be biomarkers for cerebrovascular function, and are measured in the clinic and in pre-clinical research by means of MRI-based perfusion imaging1, e.g. by arterial spin labeling (ASL) sequences providing an endogenous contrast. Anesthesia protocols in animal studies greatly influence cerebral hemodynamics. Specifically, isoflurane is a widely used volatile anesthetics having a vasodilatory effect2, thus raising baseline CBF, and reducing the dynamic range in CVR studies. Furthermore, mouse strains may respond differently to anaesthesia3. It is therefore critical to devise standardized protocols in order to provide comparable CVR estimates. Here we compare several anesthesia protocols used in the literature for CVR experiments using pseudo-Continuous ASL (pCASL). Furthermore, we compare the protocols in two mouse strains.Methods
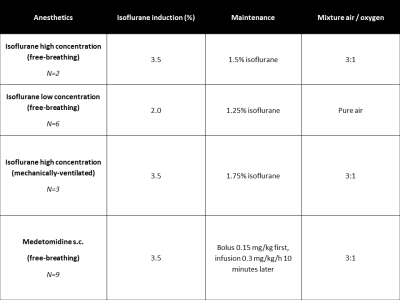
C57BL/6J and the F2 generation of a C57BL/B6J x C3H mice cross (B6C3) were anesthetized with 4 common protocols: high-dose isoflurane with normal maintenance (3.5% induction; 1.5% maintenance)3, medetomidine (after 3.5% isoflurane induction; 15mg/kg bolus, followed 10 minutes later by 30mg/kg/hr infusion)3,4, low-dose isoflurane (2.0% induction; 1.25% maintenance)5 and high isoflurane in intubated and mechanically ventilated mice (without paralysis of the animal, making it impossible to further lower isoflurane (Fig. 1). CVR was induced by administering 7.5% of CO2 in the air/oxygen mixture. A 7T Bruker PharmaScan was used with a 23-mm quadrature coil. After acquisition of T2-based anatomical scans, a pCASL scan with EPI readout was acquired in the coronal orientation (TE/TR = 16.8 ms/3,520.0 ms/3 slices, a post-labeling delay (PLD) of 300ms and a labeling time of 3,000 ms). The timeline of the CVR measurements for each anesthesia protocol was 7 minutes baseline, 7 minutes CO2 challenge, then 7 minutes return to baseline. The CBF time profiles were collected in the full brain and the cortex from the anatomical scans by applying a variational method for non-rigid multi-modal image registration6. The results are expressed as mean (standard deviation), and non-parametric Mann-Whitney tests were performed to test significant differences.Results and Discussion
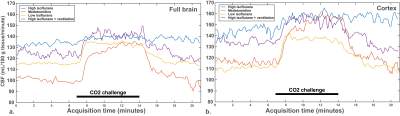
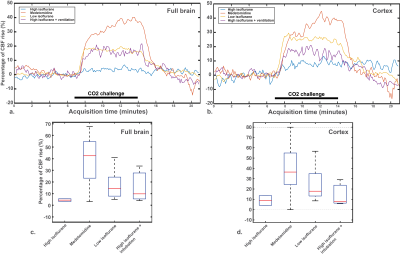
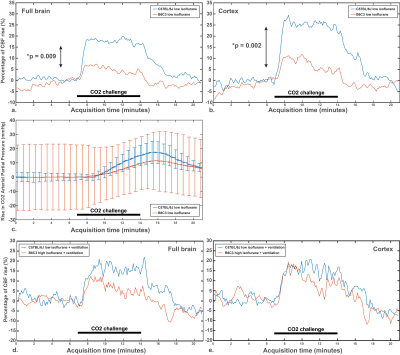
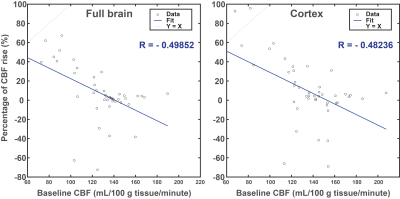
Baseline CBF values were not significantly different in C57BL/6J mice, both in the full brain and in the cortex (Fig. 2a-d). The same observation applies on the percentage of CBF rise, despite a clear CBF rise superior in the medetomidine protocol (Fig. 3a-d): high-isoflurane 4.2% (1.7%), medetomidine 38.3% (22.8%), low isoflurane 17.6% (13.1%), and high isoflurane + mechanical ventilation 15.6% (15.7%) in the full brain, and high-isoflurane 8.9% (6.7%), medetomidine 39.7% (29.1%), low isoflurane 24.9% (18.0), and high isoflurane + ventilation 14.2% (12.9%) in the cortex. The B6C3 mice could not be reliably sedated with medetomidine, thus compromising the use of medetomidine for this strain. With the free-breathing, low isoflurane protocol, a significantly lower CVR was shown in this strain vs. C57BL/6J mice (3% vs. 19% in the full brain, p=0.009; + 5% vs. 25% in the cortex, p = 0.002) (Fig. 4a and b-c), despite arterial CO2 (pCO2) being similar in the two strains (Fig. 4c). When using isoflurane and mechanical ventilation, the difference in CVR between the two strain was partially abolished (Fig. 4d-e). For C57BL/6J mice, the CVR is highly dependent on the baseline CBF, with high baseline CBF values resulting in a blunted or absent response regardless of the protocol used (Fig. 5).Conclusion
We reported on baseline CBF and CVR for 4 anesthesia protocols tested on a mixed B6C3 background vs. C57BL/6 mice. The percentage of isoflurane administered at the induction was observed to be very important, not only the maintenance. As expected, medetomidine showed the highest CVR, but was not applicable to the B6C3 strain. Others have reported on other strains being unresponsive.3 High isoflurane induction resulted in a high baseline CBF, thus blunting the CVR. Low induction isoflurane resulted in usable CVR values in C57BL/6, but not in B6C3, a strain with low sensitivity to hypercapnic stimulation7. Mechanical ventilation did not show differences in CVR in C57BL/6J compared to the low isoflurane protocol, but was shown useful in the B6C3 mice. Etomidate sedation has been suggested as a widely applicable regime in multiple strains.3 However, the large injection volumes needed exceed animal welfare guidelines. In conclusion, medetomidine is the protocol of choice in responsive strains. Free-breathing low-induction isoflurane may provide an experimentally straight-forward alternative, with CVR values comparable to those obtained in ventilated animals. As we showed that CVR is highly dependent on achieved baseline CBF values, baseline CBF should be taken into account when evaluating the CVR response. In mice with low hypercapnic ventilator sensitivity, ventilation may prove useful.Acknowledgements
This work was funded by the Netherlands Organization for Scientific Research (VIDI - Dr. Louise van der Weerd), and the Cardiovasculair Onderzoek Nederland (CVON).References
1. Maier et al 2014, Nature Med
2. Matta et al. 1999, Anesthesiol.
3. Petrinovic et al. 2016, Scientific Reports
4. Adamczak et al. 2010, Neuroimage
5. Wells et al. 2015., J Cereb Blood Flow Metab
6. Denis de Senneville et al. 2016, Phys. Med. Biol.
7. Tankersley et al. 1985, J. Appl. Physiol.
Figures