1998
Riluzole improved Energy Metabolism in AβPP-PS1 Mouse Model of Alzheimer’s Disease1NMR Microimaging and Spectroscopy, Centre for Cellular and Molecular Biology, Hyderabad, India
Synopsis
Alzheimer’s disease (AD) is a neurodegenerative disorder, characterized by degeneration of neurons leading to memory loss, deterioration in cognitive function and behavior. Despite intensive research of several decades treatment of AD is still a major challenge. Riluzole is known to be neuro-protector and regulates the function of glutamatergic neurons by reducing glutamate release and helping astroglial uptake. In this study, we have evaluated the impacts of Riluzole on the neuronal activity in the AβPP-PS1 mouse model of the AD by 1H-[13C]-NMR spectroscopy together with infusion of [1,6-13C2]glucose. The finding of improved neurometabolism in AD mice suggests riluzole improved cognitive function in Alzheimer's disease.
Introduction
Alzheimer’s disease (AD) is the most common forms of dementia, characterized by loss of memory and cognitive functions. The treatment strategies for AD have focused on decreasing Aβ-amyloid plaque load by inhibition of secretases and Aβ oligomerization, and immunotherapy. Although different approaches have been used to combat AD, there is very limited success for the treatment of the disease. Riluzole (2-amino-6-trifluoromethoxy benzothiazole), a neuroprotective molecule with antiglutamatergic action, is effective in increasing survival of amyotrophic lateral sclerosis subjects1. Although, the exact mechanism of action of the drug is yet to be identified2, it is believed that riluzole exerts it action by inhibition of Glu release through inactivation of voltage-dependent ion channels3 expression and signal transduction through Glu receptors4, and facilitation of astrocytic glutamate5. To gain better insight about the potential mechanism of riluzole's action, we have evaluated its impact on neurometabolism in the cerebral cortex and hippocampus of AβPP-PS1 mouse model of AD using 1H-[13C]-NMR spectroscopy in conjunction with infusion of [1,6-13C2]glucose6. The AβPP-PS1 mice exhibit intense plaque load and severe memory loss, which are the hallmarks of the AD7. Due to neuroprotective property of riluzole, we hypothesized that it will improve neurometabolism in AD mice.Material and Method
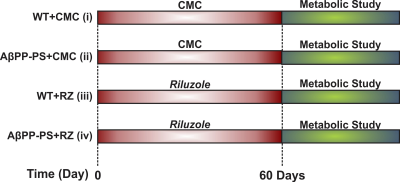
All animal experiments were performed under an approved protocol by Animal Ethics Committee of the Centre for Cellular and Molecular Biology (CCMB), Hyderabad. Male AβPP-PS1 and wild-type (WT) mice of 10 months were used in the study. Mice were divided into four groups: Group A. WT + Carboxymethylcellulose (CMC 1%) (n=6); Group B. AβPP-PS1 + CMC (n=6); Group C. WT + RZ (n=7); Group D. AβPP-PS1 + RZ (n=6). Mice in Group C and D were administered riluzole (6 mg/kg, intraperitoneal) on alternate day for 60 days while those in Group A and B received CMC (Figure 1). For metabolic measurements, urethane (1.5 g/kg, intraperitoneal) anesthetized mice were administered [1,6-13C2]glucose for 10 min through tail vein using bolus variable infusion rate8,9. Blood was collected from sinus orbital, and the head was frozen in situ into liquid nitrogen at the end of infusion. Metabolites were extracted from frozen cortical and hippocampal tissues. The concentration and 13C labeling of brain metabolites were measured in tissue extracts using 1H-[13C]-NMR spectroscopy at 600 MHz NMR spectrometer. The cerebral metabolic rate of glucose oxidation (CMRGlc(ox)) was calculated from the trapping of 13C labeled into amino acids as described previously10,11. One way ANOVA was carried to determine the statistical significance of difference in 13C labeling and cerebral metabolic rate of glucose oxidation with riluzole intervention among different groups. The post hoc Tukey honest test was carried out to further identify the statistical significance of difference between groups. All results are reported as mean±standard error of the mean.Results and Discussion
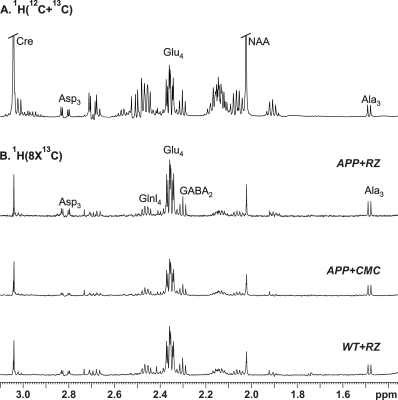
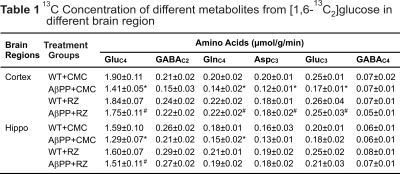
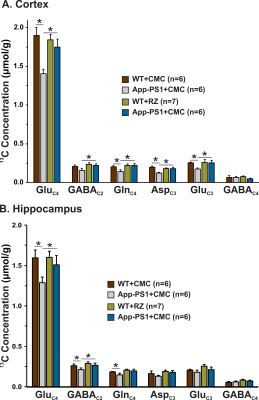
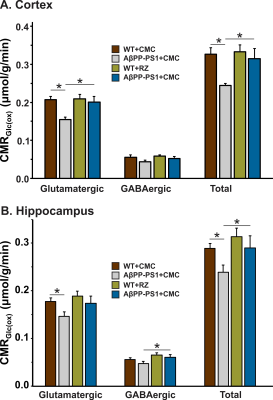
A typical 1H-[13C]-NMR spectra depicting 13C labeling of cortical metabolites are shown in Figure 2. The concentrations of 13C labeled amino acids were decreased significantly (p<0.05) in the cerebral cortex of AβPP-PS1 as compared with age-matched controls (Figure 3) indicating hypo-glucose metabolism of glutamatergic (AβPP-PS+CMC 0.15±0.01 µmol/g/min, WT+CMC 0.21±0.01 µmol/g/min, p=0.0001) and GABAergic neurons (AβPP-PS+CMC 0.04±0.01 µmol/g/min, WT+CMC 0.06±0.01 µmol/g/min, p=0.05) in AβPP-PS1 mice (Figure 4). The increased 13C labeling of cortical amino acids (Figure 2&3) following riluzole treatment in AβPP-PS1 mice indicates improved metabolic activity of glutamatergic (AβPP-PS+riluzole 0.20±0.02 µmol/g/min, AβPP-PS+CMC 0.15±0.01 µmol/g/min, p=0.007). However, the GABAergic neurons (AβPP-PS+riluzole 0.05±0.01 µmol/g/min, AβPP-PS+CMC 0.04±0.01 µmol/g/min, p=0.108) did not show significant change in the metabolism in cerebral cortex. Similar results were observed for hippocampal region (Figure 4). It has been established that neurotransmitter cycling flux is stoichiometrically coupled to neuronal glucose oxidation6. Hence, the findings of increased neuronal glucose oxidation in AβPP-PS1 mice suggest an improved excitatory and inhibitory neurotransmission in AD mice with riluzole intervention. These data suggest that riluzole intervention at the preclinical stage has potential to manage memory and cognitive function in subjects susceptible for AD.Acknowledgements
This study was supported by grant from CSIR network project BSC0208.References
1. Miller RG, Mitchell JD and Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND), The Cochrane database of systematic reviews, CD001447.
2. Cheah BC, Vucic S, Krishnan AV and Kiernan MC (2010) Riluzole, neuroprotection and amyotrophic lateral sclerosis, Current medicinal chemistry 17, 1942-1199.
3. Urbani A and Belluzzi O (2000) Riluzole inhibits the persistent sodium current in mammalian CNS neurons, The European journal of neuroscience 12, 3567-3574.
4. Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA Jr and Manji HK (2007) The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders, Neuropsychopharmacology 32, 793-802.
5. Frizzo ME, Dall'Onder LP, Dalcin KB and Souza DO (2004) Riluzole enhances glutamate uptake in rat astrocyte cultures, Cellular and molecular neurobiology 24, 123-128.
6. Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL Shulman RG and Behar KL (2004) Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 24, 972-985.
7. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT and Delon MR (1982) Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain, Science 215, 1237-1239.
8. Fitzpatrick SM, Hetherington HP, Behar KL and Shulman RG (1990) The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 10, 170-179.
9. Patel AB, Tiwari V, Veeraiah P and Saba K (2017) Increased astroglial activity and reduced neuronal function across brain in AβPP-PS1 mouse model of Alzheimer's disease, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, doi:10.1177/0271678X17709463.
10. Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG and Behar KL (2005) The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo, Proc Natl Acad Sci U S A 102, 5588-5593.
11. Saba K, Rajnala N, Veeraiah P, Tiwari V, Rana RK, Lakhotia SC and Patel AB (2017) Energetics of Excitatory and Inhibitory Neurotransmission in Aluminum Chloride Model of Alzheimer's Disease: Reversal of Behavioral and Metabolic Deficits by Rasa Sindoor, Frontiers in molecular neuroscience 10, 323.
Figures