1988
Diffusivity and the neurocognitive domains of premorbid intelligence and visuospatial memory in Pediatric Multiple Sclerosis1Stony Brook University, Stony Brook, NY, United States, 2Stony Brook University School of Medicine, Stony Brook, NY, United States, 3New York University Langone Medical Center, New York, NY, United States
Synopsis
DTI has been commonly used to study multiple sclerosis (MS) patients1-3 and many studies have correlate DTI parameters with neurocognitive functions. However, only a handful of studies4, 5 have characterized such relationships in pediatric MS patients. The goal of this study was to investigate DTI characteristics in pediatric onset MS patients and to correlate them with neurocognitive functions (intelligence and visuospatial memory).
Introduction
Fractional anisotropy (FA), axial, radial and mean diffusivity (AD, RD, MD) derived from diffusion tensor imaging (DTI) could serve as imaging biomarkers for white matter (WM) structural integrity and have hence been used to study WM demyelination and degeneration in adult and pediatric multiple sclerosis (MS) patients1-3. Many studies have correlate DTI parameters with neurocognitive functions, but a handful of studies4, 5 have characterized such relationships in pediatric or young adult MS population. The goal of this study was to investigate DTI characteristics in pediatric onset MS patients and to correlate them with intelligence and visuospatial memory.Methods
11 pediatric MS patients (age=16.5±3.8y) and 11 age/gender-matched healthy controls (HC) (age=16.8±3.8y) were scanned in a Siemens 3T MRI scanner. DTI (b=0,1000s/mm2,64-directions,2.5mm isotropic resolution, TE=95ms,TR=9600ms), B0 fieldmaps for distortion correction (3.75x3.75x2.5mm^3 resolution, TE=6.5,8.96ms, TR=900ms), 3D-T1-weighted MPRAGE sequence (1mm isotropic resolution, TE=2.44ms,TR=1920ms, TI=1100ms), and 3D FLAIR (1mm isotropic resolution, TE=402ms, TR=5000ms, TI=1800ms) images were acquired. Neuropsychological (NP) testing results for Wide Range Achievement Test (WRAT) as a measure of premorbid intelligence were available for all HC and only 9 MS, and Brief Visuospatial Memory Test (BVMT) were available in 10 HC and 7 MS.
DTI images were corrected for susceptibility-induced and eddy-current induced distortion using FSL’s Diffusion Toolbox (FDT) before FA, RD, MD and AD were mapped across the brain. WM-lesions were segmented based on their hyperintense appearance by an experienced radiologist and confirmed by our neurorardiologist. Quantitative DTI maps and lesions maps were normalized to MNI space using transformation matrices obtained from affine registration of b=0 and FLAIR images (respectively) to subject T1, and nonlinear registration of subject T1 to MNI anatomical template. Region-of-interest (ROI) analyses were performed on DTI metrics extracted from HC-WM, MS-NAWM and MS-Lesions. FSL’s RANDOMISE was used to estimate significant voxel-wise differences between controls and patients in the DTI maps. Separate GLM models were processed with WRAT and BVMT as regressors on the combined DTI maps to investigate the relationship between structural integrity and neurocognitive outcome. All voxel-wise analyses were corrected for multiple comparisons using FSL’s threshold free cluster enhancement (TFCE).
Results
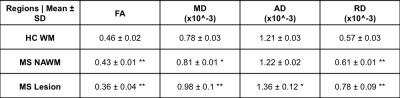
FA, RD and MD were significantly different between HC-WM and MS, and between MS-NAWM and MS-Lesions (p<0.01). AD was significantly different only between MS-NAWM and MS-Lesions (Table 1).
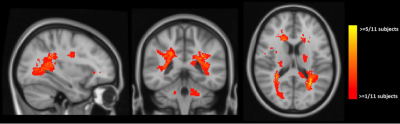
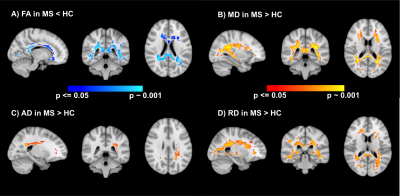
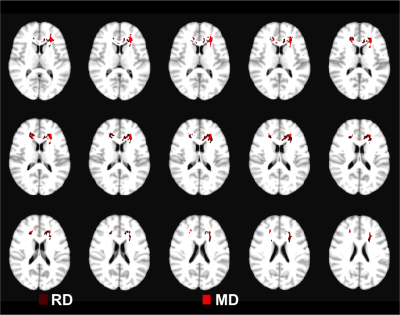
Results of voxel-wise analyses show significantly lower FA, higher MD and RD in MS compared to HC in the white-matter, especially in periventricular regions which coincide with maximum lesion incidence (Figures 1 & 2) and in corpus callosum. We also found higher AD in MS in a small cluster in the region of maximum lesions overlap.
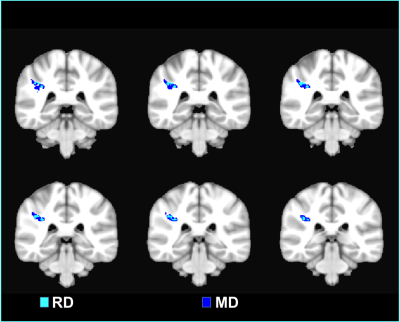
WRAT and BVMT scores were not significantly different between both populations (WRAT average: HC=109±7, MS=100±19; BVMT average: HC=31±4, MS=28±7). Linear regression of MD/ RD showed a negative correlation with WRAT in the Right Superior Longitudinal Fasciculus (SLF-R) area (Figure 3). On the other hand, a positive correlation was found between BVMT and MD/RD in the Left Anterior Thalamic Radiation and bilateral Forceps Minor (Figure 4). AD and FA did not show any significant relationship to either neuropsychological score.
Discussion
Our results from voxel-wise and ROI analyses investigating differences between HC and MS are consistent with literature1, 3. The negative correlation between WRAT and SLF-R MD/RD is expected and suggests deficits in the domain for general ability related to neurodegeneration and loss of structural integrity. Similar effects have been observed with positive FA and negative MD correlations with other cognitive domains such as IQ5 and processing speed6 in specific brain regions. On the other hand, the positive correlation between higher MD/RD in frontal white matter and higher BVMT scores is an unexpected outcome and needs further investigation.Conclusion
Results from our study confirm widespread loss of structural integrity in WM in pediatric MS as evidenced by DTI metrics. Increased mean and radial diffusivity in certain WM regions due to degeneration bear significant correlation with neuropsychological measures of intelligence and visual memory.Acknowledgements
No acknowledgement found.References
1. Absinta, M., et al., Brain macro- and microscopic damage in patients with paediatric MS. J Neurol Neurosurg Psychiatry, 2010. 81(12): p. 1357-62.
2. Sbardella, E., et al., DTI Measurements in Multiple Sclerosis: Evaluation of Brain Damage and Clinical Implications. Mult Scler Int, 2013. 2013: p. 671730.
3. Vishwas, M.S., et al., Diffusion tensor analysis of pediatric multiple sclerosis and clinically isolated syndromes. AJNR Am J Neuroradiol, 2013. 34(2): p. 417-23.
4. Rocca, M.A., et al., Is a preserved functional reserve a mechanism limiting clinical impairment in pediatric MS patients? Hum Brain Mapp, 2009. 30(9): p. 2844-51.
5. Schmithorst, V.J., et al., Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp, 2005. 26(2): p. 139-47.
6. Bethune, A., et al., Diffusion tensor imaging and cognitive speed in children with multiple sclerosis. J Neurol Sci, 2011. 309(1-2): p. 68-74.
Figures