1987
The corticospinal tract in relapsing-remitting multiple sclerosis: a preliminary tractography and fixel-based MRI analysis at ultra high-field1Anatomy and Neuroscience, University of Melbourne, Melbourne, Australia, 2Neurology, Royal Melbourne Hospital, Melbourne, Australia, 3Medicine, University of Melbourne, Melbourne, Australia, 4Florey Institute of Neuroscience and Mental Health, Melbourne, Australia
Synopsis
Lower limb disability in multiple sclerosis (MS) is likely related to axonal damage in the corticospinal tract (CST), the main motor pathway. This study aimed to compare the degree of CST degeneration to clinical motor disability using high field (7T) diffusion weighted MRI and subsequent analyses methods like tractography and fixel-based analysis. Eleven minimally impaired relapsing-remitting MS patients (1m/10f, 42±12.4yrs) were tested. Results show loss of fiber density (FD) in the subcortical white matter of the CST was associated with increased pyramidal dysfunction (puncorrected<0.05). FD could provide a useful marker of disease progression leading to loss of mobility.
Introduction
Multiple sclerosis (MS) is a progressive neurological disorder of the central nervous system associated with demyelination and axonal loss, leading to permanent disability. Mobility impairment is the most disabling symptom in MS1. Changes to lower limb disability in MS are likely related to axonal damage in the corticospinal (or pyramidal) tract, the main motor pathway.
Using the high SNR afforded by ultrahigh field (7T) MRI, diffusion MRI can be acquired with spatial resolution approaching anatomical imaging with high angular resolution within clinically feasible scan times (~10mins). This allows for analysis using higher order diffusion models such as constrained spherical deconvolution (CSD) that can estimate the fibre orientation distribution (FOD) in each voxel, together with associated axonal damage markers such as fibre density (FD) and fibre cross-section (FC) that can be analysed using “fixel-based analysis”2.
This preliminary study aimed to compare the degree of pyramidal tract degeneration (loss of FD and FC) to clinical motor disability.
Methods
Eleven relapsing-remitting MS (RRMS) patients (1male and 10 females, 42±12.4 years) were tested. All had minimal or no clinical lower limb dysfunction (as defined by the Expanded Disability Status Scale (EDSS) of less than 4, and pyramidal and cerebellar Functional Scale less than 2 or equal to 2).
All patients were imaged using a whole body Magnetom 7 Tesla MRI system (Siemens, Erlangen, Germany) with a 32-channel head coil (Nova Medical, Wilmington MA, USA). Diffusion weighted MRI was acquired using a simultaneous multi-slice 2D spin-echo EPI sequence3 (TR=7000ms, TE=72.4ms, multiband factor=2, GRAPPA=3, slices=128, 1.24mm isotropic resolution, whole brain coverage, 3 b-shells: 1000, 2000, 3000 s/mm2, 103 directions, 6 b0 images).
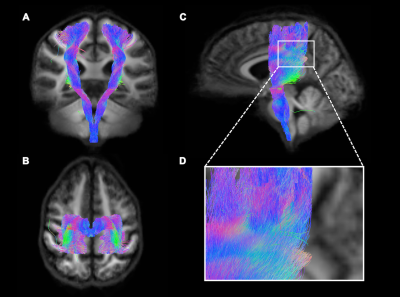
Diffusion MRI data was analysed using MRtrix 3.0. FODs were estimated for each voxel for each subject, and a population average FOD template was generated. FC and FD were computed. Whole-brain probabilistic tractrography was performed using the FOD template. The CST was identified from the whole-brain tractrogram using a seed region of interest located in the internal capsule (Fig. 1). Statistical analyses were performed on FC and FD within the CST using connectivity fixel enhancement (5000 permutations) and the pyramidal EDSS scores.
Results
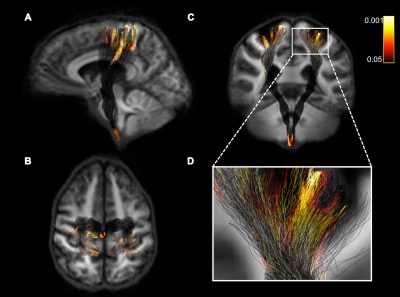
Loss of FD (but not FC) in the subcortical white matter under the sensorimotor cortex was associated with increased pyramidal dysfunction (puncorrected<0.05) (Fig. 2). No significant results were found when values were corrected for multiple comparisons. Figure 1 shows the CST fiber tracts and significant fixels represented as a tractogram and coloured with the p-value are shown in figure 2.Conclusion
Using ultra-high field 7T diffusion MRI we detected loss of FD in the cortico-spinal tract that was associated with disability. FD could provide a useful marker of disease progression leading to loss of mobility.Acknowledgements
We acknowledge the facilities, and the scientific and technical assistance of the Australian National Imaging Facility at the Melbourne Brain Centre Imaging Unit. The Australian National Imaging Facility is funded by the Australian Government NCRIS program. The work was also supported by a research collaboration agreement with Siemens Healthcare. The diffusion data was acquired with a sequence provided by CMRR, Department of Radiology, University of Minnesota, USA.References
1. Pike, J., Jones, E., Rajagopalan, K., Piercy, J. & Anderson, P. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol. 12, 94 (2012). 2. Raffelt, D. A. et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144, 58–73 (2017). 3. Vu, A. T. et al. High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. Neuroimage 122, 318–331 (2015).
Figures