1974
Microglial activation is accompanied by diffuse axonal loss in multiple sclerosis: in vivo evidence by multimodal 11C-PBR28 MR-PET and multi-shell diffusion imaging1Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3CSIC-UMH, Instituto de Neurociencias de Alicante, Alicante, Spain, 4Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden, 5Department of Radiology, Karolinska University Hospital, Stockholm, Sweden, 6Neurology, Beth Israel Deaconess Medical Center, Boston, MA, United States, 7Neurology, Massachusetts General Hospital, Boston, MA, United States, 8Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy
Synopsis
Neuropathological studies of multiple sclerosis (MS) established that diffuse microglia activation with axonal loss in the normal appearing white matter (NAWM) is a main determinant of disease progression. The in vivo study of neuroinflammation and axonal integrity is still challenging. We combined 11C-PBR28 MR-PET with multi-shell diffusion imaging to investigate neuroinflammation and microstructural abnormalities in the NAWM of MS subjects. Results showed evidence of diffuse neuroinflammation accompanied by microstructural diffusion abnormalities with decreased axonal density. The axonal density estimate from the Composite Hindered and Restricted Model of Diffusion was more sensitive than diffusion tensor imaging measures in disclosing axonal damage.
Purpose:
Neuropathological and positron emission tomography (PET) studies demonstrated neuroinflammation with microglia activation in the normal appearing white matter (NAWM) in multiple sclerosis (MS)1,2,3. Neuropathological studies also established that diffuse microglia activation is often associated with diffuse axonal loss, especially in progressive MS1. This pattern of pathology has, therefore, been considered as a main determinant of long-term disability and disease progression. Activated microglia upregulate the translocator protein 18 kDa (TSPO) expression, which can be imaged in vivo using selective TSPO PET radiotracers4. Non-invasive in vivo detection of axonal pathology in MS, however, is still challenging. Diffusion tensor imaging (DTI) studies have shown microstructural abnormalities in NAWM in MS5. DTI metrics, however, are relatively unspecific to changes in axonal microstructure. Previous studies have associated axial diffusivity to axonal loss, and radial diffusivity with myelin content6. However, diffusivity indices can be affected by crossing, branching, merging or kissing fibers among other factors. The Composite Hindered and Restricted Model of Diffusion (CHARMED)7 is an advanced multi-shell diffusion-weighted imaging method that, in contrast to DTI, provides biomarkers of tissue microstructure in which the effects of demyelination and axonal loss are disentangled by providing an estimate of the so-called restricted fraction (FR), which is a surrogate marker for axonal density. Here, in a heterogenous MS cohort, we employed a multimodal imaging approach that combined 11C-PBR28 imaging on a high resolution, integrated human MR-PET system with multi-shell diffusion imaging to investigate i) the presence of neuroinflammation in the NAWM of MS patients; ii) the microstructural diffusion abnormalities that characterize MS NAWM ii) whether the CHARMED model is a more suitable tool to detect destructive structural pathology than conventional DTI.Methods:
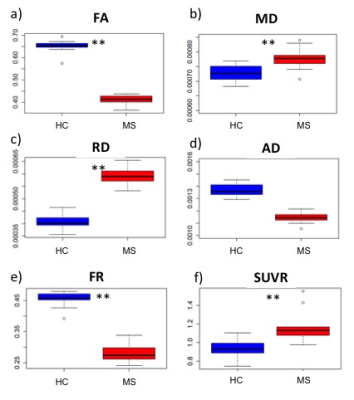
Eleven MS subjects (5 secondary progressive MS, SPMS, and 6 relapsing remitting MS, RRMS; mean±SD age=49±11 years) and 10 age- and TSPO affinity binding (as assessed by the Ala147Thr TSPO polymorphism5) matched healthy controls (HC) underwent 90-minutes of 11C-PBR28 MR-PET (Siemens BrainPET). Conventional anatomical 3 T MR scans were simultaneously acquired for: a) cortical surface reconstruction, using FreeSurfer b) MR-PET image registration. Standardized uptake value (SUV) maps were created for 60-90-minute PET frame (1.25 mm isotropic voxels) and normalized (SUVR), to take into account global differences across subjects, by a pseudo-reference region with SUV levels similar in HC and MS. On a separate session, MS subjects and 11 additional HC (mean±SD age=41±10 years) underwent 3 T imaging (Siemens Connectom scanner, 300 mT/m maximum gradient strength). Three diffusion weightings (b-values) were applied along non-collinear gradient directions: 1000 (64 directions), 5000 (128 directions) and 10000 s/mm2 (128 directions), isotropic voxel size 1.5 mm3. In addition, 28 diffusion un-weighted scans (b0 images) were acquired, interspersed throughout the diffusion-weighted scans to facilitate motion correction. Diffusion-weighted data were pre-processed using FreeSurfer and FSL tools. Pre-processing included gradient nonlinearity correction, motion correction, eddy current correction including b-matrix reorientation, after which axonal density (FR) maps were obtained through the CHARMED8 pipeline. DTI maps of mean diffusivity (MD), fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD) were obtained using Explore DTI6 and employing b=0 and b=1000 data only. Linear regression models were used to assess in the NAWM: i) 11C-PBR28 changes, in MS vs HC, ii) microstructural diffusion changes in MS vs HC and iii) the relationship between 11C-PBR28 SUVRs and diffusion parameters (DTI and CHARMED indices) in MS. Age and TSPO affinity were included as regressors when appropriate with a significant threshold of p<0.05.Results:
11C-PBR28 SUVR uptake was increased by ~26 % in the NAWM of MS group relative to HC (p=0.01). FR, FA, were significantly decreased (~38%, p=1.6 10-13, ~37%, p=2 10-16, respectively) in people with MS compared to HC. MD and RD were also significantly increased (~11%, p=0.007, ~47%, p=2 10-12) in MS compared to HC but not significant differences were found in AD, albeit it was found decreased in MS subjects relative to HC. None of the diffusion parameters correlated with the TSPO binding measure of 11C-PBR28 in the NAWM of the whole MS cohort.Discussion and Conclusion:
We found evidence of diffuse neuroinflammation and microstructural abnormalities with decreased axonal density in the NAWM of a small MS cohort. Charmed FR estimation was more sensitive than AD in detecting axonal damage. The lack of a direct correlation between 11C-PBR28 uptake and diffusion indices could indicate a multifactorial pathogenesis for axonal degeneration not necessarily linked to inflammatory demyelination. Future studies will investigate whether neuroinflammation could be directly related to axonal pathology in selective NAWM regions or in specific disease stages.Acknowledgements
This study was supported by Clafin Award; NMSS RG 4729A2/1, US Army W81XWH-13-1-0112, NIH R01NS078322-01-A1. EH is supported by an NMSS fellowship (FG-1507-05459).References
1. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705-2712.
2. Lassmann H. Mechanism of inflammation induced tissue injury in multiple sclerosis. J. Neurol.Sci. 2008;274(1-2):45-47.
3. Oh U, Fujita M, Ikonomidou VN, et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol. 2011;6(3):354-361.
4. Owen DR, Gunn RN, Rabiner EA, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. JNuclMed. 2011;52:24-32.
5. Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626-1632.
6. Song S-K, Sun S-W, Ju WK, et al. Diffusion Tensor Imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714-1722.
7. Assaf Y, Ben-Bashat D, Chapman J, et al. High b-value q-space analyzed diffusion-weighted MRI: Application to multiple sclerosis. Magn Res Med. 2002;47:115-126.
8. De Santis S, Granberg T, Ouellette R, et al. Early axonal damage in normal appearing white matter in Multiple Sclerosis: novel insights from multi-shell diffusion MRI. 2017; 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC).
Figures