1960
Progressive white matter changes in the pilocarpine-induced temporal lobe epilepsy with focal seizure rat model: A diffusion tensor imaging study1Institute of Biomedical Engineering, National Taiwan University, Taipei, Taiwan, 2Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan, 3Department of Veterinary Medicine, School of Veterinary Medicine, National Taiwan University, Taipei, Taiwan, 4Department of Neurology, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan, 5Graduate Institute of Brain and Mind Sciences, College of Medicine, National Taiwan University, Taipei, Taiwan, 6Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
A more suitable pilocarpine rat model with microinjection into the left central nucleus of the amygdala and in-vivo diffusion tensor imaging acquisitions were used to investigate progressive changes in the white matter fibers at three different time points during epileptogenesis in temporal lobe epilepsy (TLE) with focal seizure. We found transient fractional anisotropy (FA) changes in the left fimbria of the hippocampus after status epilepticus and subsequent FA changes in the left cingulum after the presence of spontaneous recurrent seizure. The results demonstrate potential imaging markers for monitoring the progression and development of TLE with focal seizure.
Introduction:
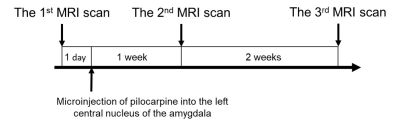
The pilocarpine rat model is widely used to investigate the progression of the epileptogenesis in temporal lobe epilepsy (TLE). Curia et al. pointed out different routes of administration of pilocarpine might influence the outcome of the pilocarpine rat model1. Specifically, recent studies found that systemic injection lead to TLE with generalized seizure2, whereas microinjection into the left central nucleus of amygdala (CeA) lead to TLE with focal seizure3. Previous diffusion MRI studies have shown widespread brain structural changes in pilocarpine-induced TLE rats4-6. However, their findings might result from generalized seizure induced by systemic administration of pilocarpine. Since TLE is the most common focal epilepsy having large-scale white matter (WM) alterations in human7, the present longitudinal study performed a more suitable pilocarpine rat model with the CeA microinjection and in-vivo diffusion tensor imaging (DTI) acquisitions to investigate progressive changes in the WM fibers at three different time points in TLE with focal seizure (figure 1), i.e. 1 day before CeA microinjection (baseline), 1 week after CeA microinjection (after status epilepticus for 1 week), and 3 weeks after CeA microinjection (the presence of spontaneous recurrent seizure). We hypothesized that in-vivo DTI could detect progressive microstructural changes in the WM during epileptogenesis that might help us to gain knowledge of mechanisms underlying seizure propagation in TLE with focal seizure.Methods:
Animals: Fifteen male Sprague-Dawley rats (weighing=250-300 grams) were used in this study. Eight TLE rats were treated with left CeA (the Paxinos and Watson rat atlas coordination: AP, 2.8 mm from bregma; ML, 4.2 mm; DV, 7.8 mm relative to bregma) microinjection of pilocarpine3 (total volume=1 μl); seven control rats were treated with left CeA microinjection of normal saline (total volume=1 μl). Six screw EEG electrodes were respectively placed on the bilateral frontal, parietal, and occipital lobes to monitor epileptiform activity after the left CeA microinjection of pilocarpine. MRI data acquisition: MRI scanning was performed on a 7 Tesla scanner with a bore 30 cm in diameter (Bruker Biospec, Ettlingen, Germany). T2W imaging was performed using a RARE sequence (26 coronal slices, thickness = 1 mm, TR/TE = 2500/26.7 ms, matrix size = 200×200, FOV = 30×30 mm, average = 6). DTI was performed using a 2D gradient EPI sequence (30 axial slices, thickness=0.4 mm, TR/TE=7500/34 ms, matrix size=75×75, FOV=30×30 mm, average=40). DTI data preprocessing and analysis: Data preprocessing included: skull stripping of diffusion weighted images and using the DSI studio to generate individual fractional anisotropy (FA) maps. At each time point, we used an SPM 12b function of the pairwise longitudinal registration to create a study-specific template (SST) from all individual FA maps and obtained deformation matrices. By multiplying these deformation matrices, all individual FA maps were normalized to an SST space at each time point. Finally, we performed voxel-wise two-sample t-test to compare normalized FA maps between TLE and control groups to detect microstructural changes in the WM at each time point. All anatomical locations of FA maps were confirmed by referring to a DTI atlas of the Sprague-Dawley rat8.Results:
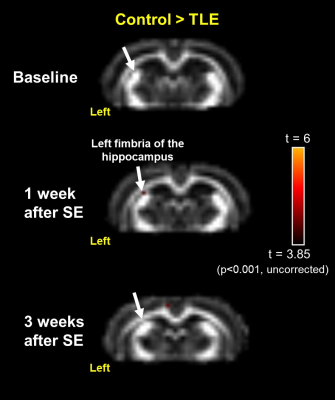
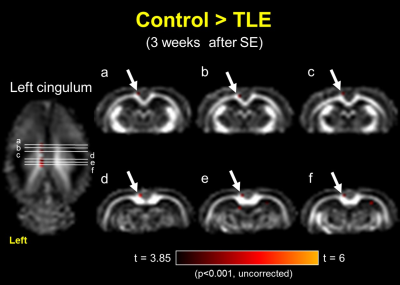
After the left CeA microinjection of pilocarpine, the predominant epileptic EEGs were immediately recorded from the left parietal electrode, indicating the left CeA microinjection successfully induced focal epilepsy in rats. The significant results of between-group comparisons with FA maps at three time points are as follows: after the left CeA microinjection for 1 week (the 2nd time point), the left fimbria of the hippocampus (FH) revealed FA reductions in TLE group as compared to the control group (figure 2, p < 0.001, uncorrected); when we observed spontaneous recurrent seizure at the 3rd time point in the TLE group, FA reduction was present in the left cingulum (figure 3, p < 0.001, uncorrected) and absent in the left FH.Discussion:
Our findings revealed the progression of the WM microstructural changes during epileptogenesis in TLE with focal seizure. To begin with, the impaired FH might be induced by status epilepticus and following myelin and hippocampal damages5,6,9, because the FH is a major efferent pathway of the hippocampus. After a latent period of 3 weeks for developing spontaneous recurrent seizure in the chronic stage of TLE, the FH was followed by recovery and more WM impairments occurred in the left cingulum. It is speculated that status epilepticus might induce transient FA changes in the FH5 and spontaneous recurrent seizure might contribute to subsequent FA changes in the cingulum, indicating a potential seizure propagation pathway.Conclusion:
The present study might provide potential imaging markers for monitoring the progression of epileptogenesis in TLE with focal seizure.Acknowledgements
No acknowledgement found.References
1. Curia G, Longo D, Biagini G, et al., The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008 Jul 30;172(2):143-57.
2. Jou SB, Kao IF, Yi PL, et al., Electrical stimulation of left anterior thalamic nucleus with high-frequency and low-intensity currents reduces the rate of pilocarpine-induced epilepsy in rats. Seizure. 2013 Apr;22(3):221-9.
3. Yi PL, Lu CY, Cheng CH, et al., Activation of amygdala opioid receptors by electroacupuncture of Feng-Chi (GB20) acupoints exacerbates focal epilepsy. BMC Complement Altern Med. 2013 Oct 29;13:290.
4. Kuo LW, Lee CY, Chen JH, et al., Mossy fiber sprouting in pilocarpine-induced status epilepticus rat hippocampus: a correlative study of diffusion spectrum imaging and histology. Neuroimage. 2008 Jul 1;41(3):789-800.
5. van Eijsden P, Otte WM, van der Hel WS, et al., In vivo diffusion tensor imaging and ex vivo histologic characterization of white matter pathology in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia. 2011 Apr;52(4):841-5.
6. Salo RA, Miettinen T, Laitinen T, et al., Diffusion tensor MRI shows progressive changes in the hippocampus and dentate gyrus after status epilepticus in rat-histological validation with Fourier-based analysis. Neuroimage. 2017 May 15;152:221-236.
7. Gross DW, Concha L, Beaulieu C, Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006 Aug;47(8):1360-3
8. Veraart J, Leergaard TB, Antonsen BT, et al., Population-averaged diffusion tensor imaging atlas of the Sprague Dawley rat brain. Neuroimage. 2011 Oct 15;58(4):975-83.
9. Norton WT, Poduslo SE, Myelination in rat brain: changes in myelin composition during brain maturation. J Neurochem. 1973 Oct;21(4):759-73.
Figures