1959
Comprehensive assessment of white matter microstructural integrity and its change across lifespan in patients with tuberous sclerosis complex1Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan, 2Department of Pediatrics, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan, 3Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
In previous studies, white matter microstructural integrity and its lifetime change in patients with tuberous sclerosis complex (TSC) were not clearly identified. Therefore, we performed diffusion spectrum imaging using whole-brain tract-specific analysis to measure the generalized fractional anisotropy (GFA), and built an age-GFA quadratic linear model to investigate 76 major white matter tract bundles between TSC and healthy control groups. Twenty tract bundles showed a group effect with substantially lower GFA in childhood and older adulthood in patients with TSC. Our results suggest that TSC might pose detrimental effects on microstructural integrity in the developmental and aging periods of life.
Introduction
Tuberous sclerosis complex (TSC) is a genetically determined multisystem disorder that may affect any human organ system, including the central nervous system. The majority of individuals with TSC have neuropsychological, behavioral, intellectual, scholastic, and psychiatric difficulties1. The cortical tubers and white-matter abnormalities in children and adolescents with TSC have been studied using region-of-interest diffusion tensor imaging analyses with small sample sizes2, 3. However, comprehensive assessment of the whole brain white matter integrity and its change across lifespan are still lacking. Therefore, we used whole-brain tract-specific analysis of diffusion spectrum imaging (DSI) data to investigate the microstructural integrity and its lifespan change of 76 major white matter tract bundles in patients with TSC.Methods
Participants: Thirty-eight patients with TSC who were over 5 years old and had no medication treatment, no dilatation of bilateral lateral ventricles, no large intra-ventricular tumors, and no cysts (age: 23.9 ± 11.9, range: 7-54 years old, 14 males and 24 females) and 38 age- and sex-matched healthy controls (age: 23.9 ± 12.0, range: 7-56 years old, 14 males and 24 females) were recruited in the study. MRI data acquisition: The data of T1-weighted images and DSI were acquired on a 3T MRI system (TIM Trio, Siemens, Erlangen) with a 32-channel phased array coil. T1-weighted imaging utilized a 3D magnetization-prepared rapid gradient echo pulse sequence (TR/TE=2000/3 ms, flip angle = 9°, FOV=256×192×208 mm^3, matrix size=256×192×208, and spatial resolution=1×1×1 mm^3). DSI utilized a pulsed gradient twice-refocused spin-echo diffusion echo planar imaging sequence using a total of 102 diffusion encoding gradients with the maximum diffusion sensitivity bmax = 4000 s/mm^2. (TR/TE=9600/130 ms, FOV=200×200 mm^2, matrix size=80×80, slice numbers=56, and slice thickness=2.5 mm). Data analysis: We used whole tract-based automatic analysis (TBAA) to obtain a 2D connectogram for each DSI dataset, which provided generalized fractional anisotropy (GFA) profiles of 76 white matter tract bundles4. An age-GFA quadratic linear model was built to compare the group difference in mean GFA of each tract bundle between TSC and HC groups.Results
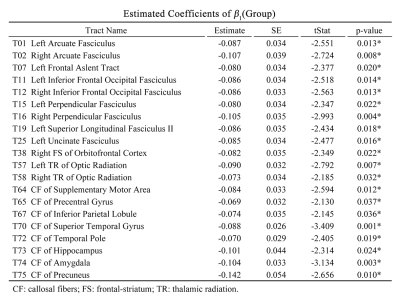
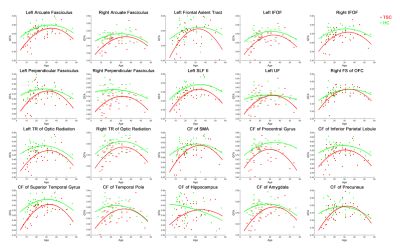
A group effect between TSC and HC and reduced mean GFA in patients with TSC was found in 20 tract bundles (Table 1, p uncorrected), including bilateral arcuate fasciculus (AF), the left frontal aslent tract, bilateral inferior frontal occipital fasciculus (IFOF), bilateral perpendicular fasciculus, the superior longitudinal fasciculus II (SLF II), the left uncinate fasciculus (UF), the right frontal-striatum (FS) of the orbitofrontal cortex (OFC), bilateral thalamic radiation (TR) of optic radiation, the callosal fibers (CF) of supplementary motor area (SMA), CF of precentral gyrus, CF of Inferior parietal lobule, CF of superior temporal gyrus, CF of temporal pole, CF of hippocampus, CF of amygdala, and CF of precuneus. In the fitted age-GFA curves, the TSC group had substantially lower GFA values than the HC group in the childhood and older adulthood with the smallest difference in the middle age (30-40 years) in most of tract bundles (Figure 1).Discussion
Previous studies have revealed the functions of the tract bundles. The AF is responsible for recognizing language and responding appropriately5, and the IFOF is associated with executive function and global cognitive function6. The SLF II is important for the maintenance of attention, while the UF is involved in emotion processing, memory and language functions7. CF of the superior temporal gyrus is significant for processing auditory information, social cognition, and regulation of behavior2. CF of the temporal pole, hippocampus, amygdala, and precuneus belong to the limbic system group, which are primarily involved in emotion and memory function8. The results of reduced mean GFA in 20 tract bundles might be implicated in the abnormality in behavior, cognitive function, language, memory, and emotion in patients with TSC. Furthermore, this study reveals an atypical pattern of GFA change across lifespan in altered tract bundles. Patients with TSC exhibit delayed development in the childhood, approaching to HC in the middle age (30 to 40 years old), and rapid degeneration in the older adulthood. The pattern suggests that TSC poses detrimental effects on developmental and aging periods of life. A limitation of the study was the relatively small sample size above 50 years old, causing unstable age-GFA curves in a few tract bundles. Nonetheless, most of the tract bundles showed the same trend, supporting the validity of our observations.Conclusion
The atypical age-GFA patterns in 20 altered tract bundles suggest the loss of white matter microstructural integrity in patients with TSC and possible disease-related detrimental effects on developmental and aging processes of life. Future work involving follow-up of individual patients is warranted to validate our observation.Acknowledgements
No acknowledgement found.References
1. David J. Kwiatkowski, et al. Tuberous sclerosis complex: genes, clinical features and therapeutics. Wiley: Wiley-Blackwell. 2010.
2. Krishnan ML, et al. Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol. 2010; 42(2):101-106.
3. Karadag D, et al. Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol. 2005; 35(10):980-983.
4. Chen, Yu-Jen, et al. Automatic whole brain tract-based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Human brain mapp. 2015; 36(9):3441-3458.
5. Leclercq D, et al. Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. J Neurosurg. 2010; 112(3):503-511
6. Peters BD, et al. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 2014; 75(3):248-256.
7. Schmahmann JD, et al. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008; 1142:266-309.
8. Wanda Webb PhD, et al. Neurology for the speech-language pathologist. ELSEVIER. 2016.