1957
Case Study: Evaluation of White Matter Disorganization in Temporal Lobe Epilepsy1UBC MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 2Radiology, University of British Columbia, Vancouver, BC, Canada, 3Neurology, University of British Columbia, Vancouver, BC, Canada, 4Laval University, Quebec City, QC, Canada
Synopsis
Temporal lobe epilepsy (TLE) assessment on MRI is limited to qualitative analysis in the clinical environment. Diffusion Tensor Imaging has been used to interrogate white matter changes in TLE while Myelin Water Fraction has not. With this case study we compare diffusion tensor imaging with myelin water imaging in a non-human primate (NHP) with TLE and a healthy control to assess if the two methods are complementary in evaluating white matter disorganization.
Introduction
MRI evaluation of Temporal Lobe Epilepsy (TLE) in clinical practice relies on a qualitative assessment of the hippocampus to confirm sclerosis1. Quantitative MRI may be helpful in clinical decision-making as not all epileptogenic foci are visible on conventional qualitative MRI and not all TLE patients are seizure-free following temporal lobectomy1-2. Investigating TLE with Diffusion Tensor Imaging (DTI) has demonstrated white matter changes in both the epileptogenic temporal lobe and in related structures in both hemispheres2-5. DTI in conjunction with Myelin Water Fraction (MWF) may be a useful combination for the evaluation of white matter disorganization in TLE.Methods
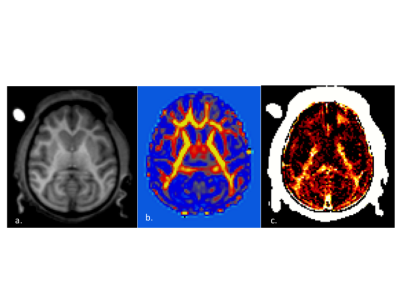
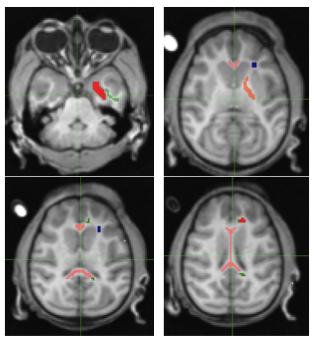
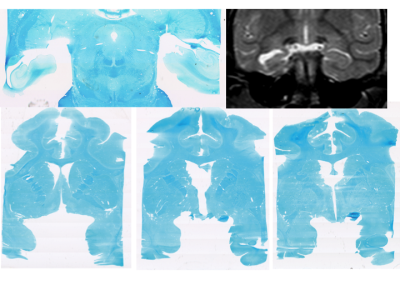
An adolescent Rhesus NHP that exhibited spontaneous absence seizures and an age and gender matched control were scanned using a 3T Philips Achieva MRI (Best, the Netherlands). DTIhardi60 (TR 4300ms, TE 60ms, b0 and 1000, voxel 1.67x1.67x1.8) and a 32 Echo GRASE MWF acquisition (TR 1000ms, Echo Spacing 10ms, voxel 0.96x0.95x5mm slice recon to 2.5mm) were performed along with T1, T2 and FLAIR anatomical imaging. The anatomical images from the affected and control NHP were compared and evaluated by a MR radiologist. Fractional Anisotropy (FA), Axial Diffusivity (Dax), Radial Diffusivity (Drad) and Mean Diffusivity (MD) maps were obtained, along with MWF maps (Fig.1). The following regions of interest were manually delineated: Corpus Callosum (CC) Genu/Body/Splenium, Corona Radiata (CR), Cingulate Gyrus (CG), Frontal white matter (FWM), Internal Capsule (IC) and Temporal Lobe white matter (TWM)6(Fig.2). Limited Luxol fast blue stained brain sections were obtained, however optical density values were not available at the time of this submission.
Results
The conventional MRI showed anatomical findings consistent with TLE in humans, with moderate enlargement of the left temporal portion of the lateral ventricles with atrophy of the left hippocampus and medial temporal lobe with thinning of the grey and white matter but did not show any focal signal abnormalities. Histological observation confirmed the atrophy of the left hippocampus, with an observable decrease of Luxol fast blue staining in the external capsule and frontotemporal association tracts (Fig.3).
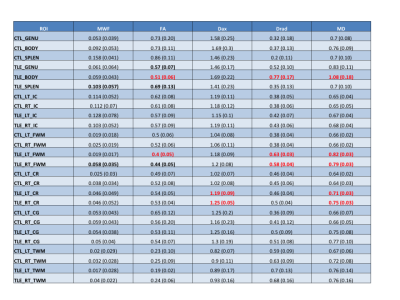
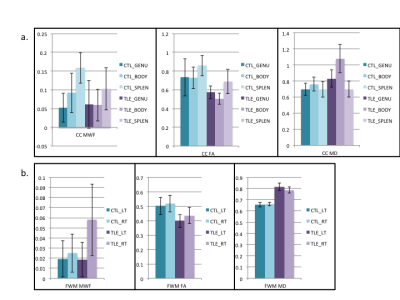
There was a trend towards decreased MWF in the TLE Splenium and an increase in the contralateral frontal white matter in the TLE NHP versus the control. The MWF in the control NHP was consistent with previous work showing higher values in the splenium7.
There was a decrease in FA of the body of the CC with an increased MD and Drad in the TLE NHP versus the control. FA showed trends towards decreases in the genu and splenium. The ipsilateral FWM showed decreased FA, with less of a decrease in the contralateral FWM. There were also increases in Dax and Drad in the ipsilateral FWM. MD was increased bilaterally in the FWM and CR with the CR also showing increased axial diffusivity. There were no significant findings in the CG, IC or TWM (Fig.4).
Discussion
The diffusivity changes in the CR could be due to the proximity of other temporal lobe association fibres. The decreased mean FA with increased MD in the CC are consistent with previous DTI findings4 and the trend towards decreased MWF in the TLE NHP indicates that MWF could offer further insight into the nature of white matter disorganization in TLE. The FWM in both hemispheres had increased WM disorganization as shown with FA with an increased MWF in the contralateral hemisphere only (Fig.5). The nature of these findings requires further study, but could be the result of frontotemporal tract damage and be compensatory in the case of the MWF increase.
The limitations of this study include the large voxel size of the MWF acquisition, the small size of the brain of the adolescent NHP and the nature of case study comparison. Smaller white matter tracts such as the external capsule and frontotemporal association tracts, interrogated on previous TLE DTI studies2-3, could not be reliably delineated. Visual observation of the histological samples did reveal the loss of Luxol fast blue stain in the external capsule and the uncinate fasiculus3.
Conclusion
Temporal lobe epilepsy affects multiple WM tracts in the brain, and has been previously studied with advanced DTI techniques but not in conjunction with MWF. This case study suggests that these techniques may be complementary in assessing white matter disorganization in TLE. Quantitative MRI techniques other than DTI may be needed to inform clinical decision making as DTI findings have not conclusively been linked to poorer clinical outcomes in TLE9. Further studies are needed in a human population to investigate the WM changes in TLE with MWF, particularly the smaller white matter tracts associated with the temporal lobe.Acknowledgements
I would like to acknowledge Brain Canada as the funding source for this project.References
1. A.Struck, M.Westover. Variability in Clinical Assessment of Neuroimaging in Temporal Lobe Epilepsy. Seizure 2015 Aug 30: 132-135.
2. Raúl Rodríguez-Cruces, Luis Concha. White Matter in Temporal Lobe Epilepsy: Clinico-Pathological Correlates of Water Diffusion Abnormalities. Quant Imaging Med Surg 2015;5(2):264-278
3. Otte et al. A Meta-analysis of White Matter Changes in Temporal Lobe Epilepsy as Studied With Diffusion Tensor Imaging. Epilepsia, 53(4):659–667, 2012
4. Lyra et al. Corpus Callosum Diffusion Abnormalities in refractory epilepsy associated with hippocampal sclerosis. Epilepsy Research Vol.137:112-118, 2017
5. Urbach et al. Bilateral Cingulum fiber reductions in temporal lobe epilepsy with unilateral hippocampal sclerosis. European Journal of Radiology Vol.94:53-57, 2017.
6. Paxinos, Huang, Toga, The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; 1st edition (November 18, 1999)
7. Stikov et al. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage Vol.118 397-405, Sept.2015
8. Gross et al. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 2006 Aug 47(8) 1360-3.
Figures