1953
Hemispheric Regional Based Analysis of Diffusion Tensor Imaging and Diffusion Tensor Tractography in Patients with Temporal Lobe Epilepsy1Thomas Jefferson University, Philadelphia, PA, United States, 2Temple University, Philadelphia, PA, United States, 3University at Buffalo, Buffalo, NY, United States
Synopsis
Diffusion tensor imaging and diffusion tensor tractography help to better understand the pathological alterations in white matter structures, and in tracing axonal pathways involved in patients with temporal lobe epilepsy.
Background and Purposes:
Imaging in the field of epilepsy has been challenging, specifically in identifying the seizure focus as a resectable area for neurosurgical procedures [1]. This has created the opportunity for application of newer approaches and imaging innovation in the field of epilepsy, such as with Diffusion Tensor Imaging (DTI) and diffusion tensor tractography (DTT). In this study, we aim to evaluate the use of DTI and DTT as a predictive model in patients with temporal lobe epilepsy (TLE). A hemispheric based analysis approach was used to compare the pathologic hemisphere to the healthy hemisphere in TLE patients.Methods
Subjects: In this retrospective mono-center study, a total of 25 patients with TLE (13 males, 12 females, 22-57 age range) underwent either anterior temporal lobectomy (ATL) or Selective Laser Amygdalohippocampectomy (SLAH). On pre-operative MRI, 15 TLE patients were diagnosed with left mesial temporal sclerosis (LMTS), 7 were diagnosed with right MTS (RMTS), and 3 were found to be normal, based on the pre-operative MRI. Additionally, patients were diagnosed based on the pre-operative PET scan which revealed 11 of the 25 TLE patients to have hyperactive areas in the left temporal lobe (TL), 6 in the right TL, while 2 had normal PET findings. PET findings for 6 patients were not available. Of the participants, 14 underwent SLAH while 11 underwent ATL.
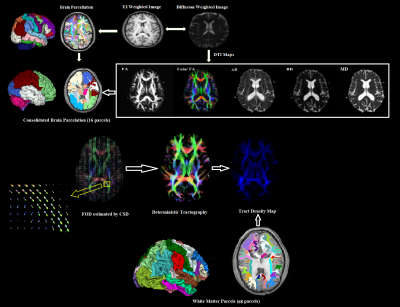
Imaging: DTI images were acquired axially in the same anatomical location prescribed for the T1-weighted images. The T1-weighted imaging parameters used were: FOV=24.0cm, voxel size=1.0×1.0×1.0mm3, matrix size=512×512, TR=12ms, TE=6ms and slice thickness=1mm. The DTI parameters used were: FOV=23.0cm, number of directions=15, number of reference scans (b0)=1, b=850s/mm2, voxel size=1.8×1.8×2.0mm3, matrix size= 128×128, TR=8.9s, TE=62ms, number of averages=1.
DTI post-processing: The raw data set of the diffusion volumes were first corrected for eddy current distortions and motion artifacts. Each directional diffusion image was aligned to the b0 volume (reference image) based on the 3D rigid body registration algorithm. Various DTI indices such as fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) were generated. The FA, MD, RD and AD maps of the TLE patients were then co-registered to the brain parcellation map in the freesurfer space based on affine transformation algorithm and normalized mutual information as cost function (Figure 1). FreeSurfer provides parcellation of anatomical regions of cortices and subcortical regions inboth the hemispheres. 16 consolidated cortical and subcortical regions were selected as regions of interest (ROIs) by a functional neurosurgeon and DTI values for each ROI were calculated and compared with the corresponding ROI in the opposite hemisphere. Additionally, tract density imaging (TDI) of 68 white matter parcels were generated using fiber orientation distribution (FOD) based deterministic fiber tracking and compared with contralateral side of the brain in each epileptic group (LMTS, RMTS and MRI normal). FOD was estimated using the constrained spherical deconvolution (CSD) model implemented in MRtrix.