1952
MRI and CT derived 3D-printed patient specific brain model for localizing depth elecrodes for epilepsy surgery planning1Radiology, The Medical College of Wisconsin, Milwaukee, WI, United States, 2Pediatric Neurosurgery, The Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurosurgery, The Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
We present a method for creating a patient specific, 3D printed model of depth electrode location in an epilepsy patient. We utilized a pre-surgery structural MRI scan and a post-electrode placement CT, which were aligned, and combined to visualize a cortical anatomy and electrode position. 3D models were then generated, edited, and 3D-printed to provide a visual and physical aid for surgical planning.
Introduction
Intracranial EEG recording is often required with intractable epilepsy cases to determine the resection site. Subdural electrodes have been shown to miss or even incorrectly lateralize seizures detectable by depth electrodes1. The draw back to depth electrodes versus subdural electrodes is the difficulty in reading where precisely each electrode is while in surgery from black and white 2D images that cannot capture brain tissue detail. 3D models have been shown to improve surgical outcome in neurosurgery for both aneurysm and brain malformations2,3. Graspable 3D models have further been argued to be more helpful in surgical outcomes, patient interactions, learning and teaching, and medical research4. We have developed a technique for visualizing depth electrodes in high-resolution 3D printed models for the purpose of aiding neurosurgeons in preparing for resective epilepsy surgeries.Methods
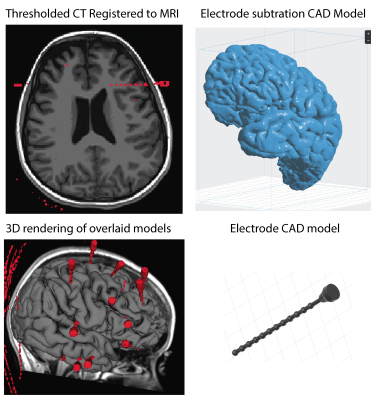
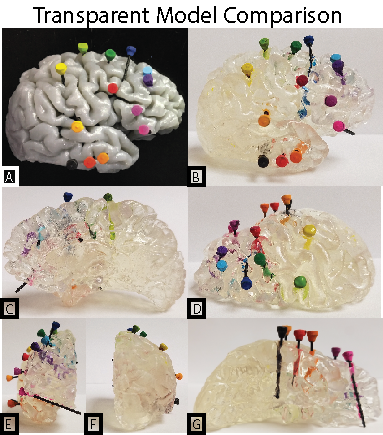
For the creation of precise models an axial 3D T1 MRI was acquired pre-surgical insertion of depth electrodes. After stereo-electroencephalography (SEEG) and depth electrodes were placed a CT scan was obtained to identify electrode location. Cortical segmentation was performed using freesurfer on the anatomical MRI5. The CT was then thresholded and binarized. A surface model was then wrapped around the binarized CT using slicer (www.slicer.org) and saved as an STL file. Both the cortical surfaces and the electrode models were then loaded into Blender (www.blender.org) for additional 3D modelling. The CT imaged electrodes were refined and modeled in this program to enhance the visibility. Electrode models were hollowed out from the MRI model of the brain tissue using a boolean modifier. The refined brain model was separated by hemisphere exported to Preform (www.formlabs.com) software for 3D printing. The Formlabs printer (Form 2) is a stereo lithography printer, using high-resolution ultraviolet laser on clear photopolymer resin. The electrodes were printed out separately as individual electrode stick models (Figure 1), which were then color coded to the surgeon’s reference list. The printed model was then processed to remove supports and add a clear finish to enhance model clarity (Figure 2).Results
The 3D model accurately showed the depth electrode placement in a graspable and manipulateable format. With each hemisphere printed separately, the electrodes were more visible deeper in the brain.Discussion
Seizure monitoring with depth electrodes is a less invasive technique than the placement of large electrode grid arrays. Imaging to determine the location of each electrode is however critical for surgical planning. We present a method for generating a graspable 3D model based on MRI and CT. Our surgeons are enthusiastic about this technique, and have found the models most helpful when printed to actual size. This is however a drawback to cost and print time. Models printed in clear resin still obstruct some clarity to electrodes that are half way into the hemisphere due to the distortion the curved gyri impose.Conclusion
We have found that 3D printed models aid in a surgeon’s ability to visualize the location of depth electrodes for surgical planning. This technique can be easily replicated with the appropriate scans to create an accurate model for surgeons to work and plan from.Acknowledgements
No acknowledgement found.References
1. Sperling MR, O'Connor MJ. Comparison of depth and subdural electrodes in recording temporal lobe seizures. Neurology. 1989;39(11):1497-1504.
2. Wurm G, Tomancok B, Pogady P, Holl K, Trenkler J. Cerebrovascular stereolithographic biomodeling for aneurysm surgery. Technical note. J Neurosurg. 2004;100(1):139-145.
3. Giesel FL, Hart AR, Hahn HK, et al. 3D reconstructions of the cerebral ventricles and volume quantification in children with brain malformations. Acad Radiol. 2009;16(5):610-617.
4. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341.
5. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207.
Figures