1943
Use of Functional MRI to assess the differences of STN and GPI Deep Brain Stimulation in Parkinson Disease1Neuroscience and Experimental Therapeutics, Albany Medical College, Albany, NY, United States, 2GE Global Research Center, Niskayuna, NY, United States, 3Neurosurgery, Albany Medical College, Albany, NY, United States, 4GE Global Research Center, Bangalore, India, 5Neurology, Albany Medical College, Albany, NY, United States
Synopsis
Deep brain stimulation (DBS) of both the subthalamic nucleus (STN) and globus pallidus interna (GPi) are well-recognized effective treatments for Parkinson’s disease (PD). The mechanism of DBS and network responses produced by stimulation of these targets remains unknown. Conditional labeling of DBS now allows fMRI to be performed in the ON state. We examine whether GPI DBS and STN DBS affect blood oxygen level dependent (BOLD) brain activation/deactivation patterns similarly. Results show that both types of DBS activate the thalamus and deactivate the primary motor cortex; while the STN cohort showed activation in the cerebellum, an opposite effect was apparent in the GPi cohort.
Introduction
Deep brain stimulation (DBS) is a well-accepted treatment of Parkinson’s disease (PD). Both the subthalamic nucleus (STN) and the globus pallidus interna (GPi) have been used as targets. Which target is used for implantation depends on the multi-disciplinary teams’ assessment of risk versus benefit, with STN having more adverse neuropsychological effects, but also a greater chance of medication reduction. Whether network activity differs with target remains unknown. A recent conditional change in DBS labeling allows MRI to be performed with DBS turned ON. We present a preliminary study on how STN or GPI DBS for PD affects blood oxygen level dependent (BOLD) activation/deactivation.Methods
Fifteen PD subjects with clinically optimized DBS programming underwent fMRI exams. Following the acquisition of T1-weighted anatomical scans, task-based fMRI imaging volumes were obtained while the DBS device was cycling between on and off states in 30s increments. To ensure that fMRI cycling and subject programmer cycling were synchronized, an electronics box coupled to electrocardiogram leads were integrated into the workflow1. Unified Parkinson’s disease rating scale-III (UPDRS-III) scores with DBS ON and OFF were documented and medications were administered as per patient routine. Model-based voxel-wise General Linear Model (GLM) was used to determine which regions were altered by the DBS being turned ON with a threshold of p<0.005 (cluster threshold=50). To be able to visualize areas of commonality between the STN and GPI cohorts, group analysis was performed. For this, the previous outputs of the GLM analysis were used to perform a one-sample t-test to determine the voxel-wise statistics using SPM12.Results
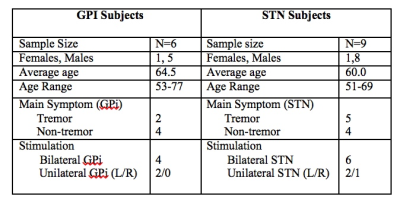
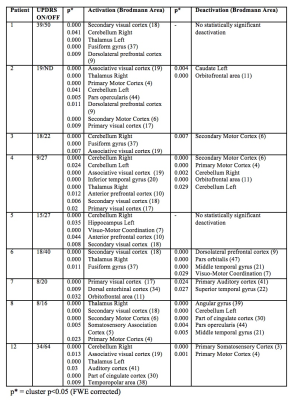
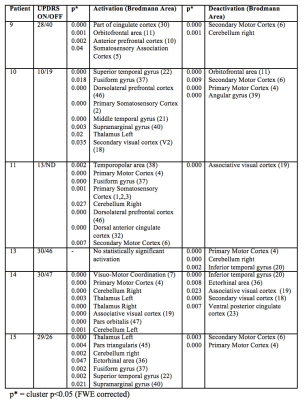
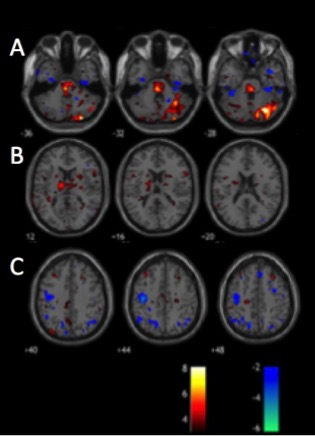
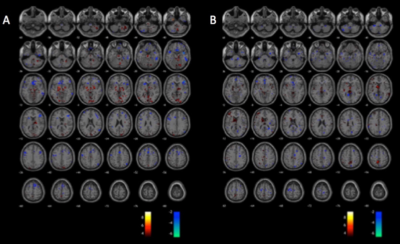
Patient demographics including age, male/female ratio, type of DBS stimulation (GPI vs. STN) and the predominant symptoms are displayed in Figure 1. The individual activation/deactivation results under the GLM analysis is summarized in Figure 2 (STN) and Figure 3 (GPi). While there is a large amount of variability within the cohorts, areas such as the thalamus, cerebellum, prefrontal cortex, primary and secondary motor cortex appear frequently, which, holds consistent with previous findings looking at regions of activation utilizing DBS 2, 3. Group analysis of the STN cohort showed activation in the thalamus and cerebellum and deactivation in the supplementary motor area (SMA) and primary motor cortex (M1) (Fig. 5). Group analysis of the GPi cohort showed activation in the thalamus, caudate and deactivation of the cerebellum and M1 (Fig. 5).Discussion
From this data we speculate that both STN and GPi DBS have a similar effect on the nigrostriatal pathway, ultimately resulting in thalamic activation and M1 deactivation, presumably normalizing motor output in PD. While both types of DBS showed to regulate the thalamic and motor cortex, there were discrepancies within the cerebellum, consistent with other studies that have contradictory findings4-6. Future studies will be powered to confirm effects on the cerebellum, which could account for differing effects on tremor control.Conclusion
In conclusion, we were able to utilize fMRI to visualize (de)activation patterns with STN and GPi DBS. These findings will allow us to better understand how DBS is able to modulate the nigrostriatal pathway to improve the motor impairment caused by Parkinson’s disease. Understanding the differences between STN and GPi DBS may provide assistance in determining which nuclei is the most appropriate for DBS implantation and finding the optimal target for the patient.Acknowledgements
No acknowledgement found.References
1. Fiveland E, Madhavan R, Prusik J, et al. EKG-based detection of deep brain stimulation in fMRI studies. Magn Reson Med 2017.
2. Phillips MD, Baker KB, Lowe MJ, et al. Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus--initial experience. Radiology 2006;239:209-216.
3. Cilia R, Marotta G, Landi A, et al. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case-control study. Clin Neurol Neurosurg 2009;111:140-146.
4. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage 2007;35:222-233.
5. Bradberry TJ, Metman LV, Contreras-Vidal JL, et al. Common and unique responses to dopamine agonist therapy and deep brain stimulation in Parkinson's disease: an H(2)(15)O PET study. Brain Stimul 2012;5:605-615.
6. Sutton AC, O'Connor KA, Pilitsis JG, Shin DS. Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain structure & function 2015;220:3595-3609.
Figures