1939
A Cycling Exercise Study of Parkinson’s Disease: The Effect of Exercises on Motor Cortex Functional Connectivity Revealed by Resting State FMRIJian Lin1, Katherine A Koenig1, Erik Beall2, Mark J Lowe1, Amy E Jansen3, Amanda L Penko3, and Jay Alberts3
1Radiology, Cleveland Clinic, Cleveland, OH, United States, 2Hema Imaging LLC, Minneapolis, MN, United States, 3Biomedical Engineering, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Parkinson’s
disease (PD) is a progressive neurological disorder which produces a general
poverty of movement. Lower extremity forced exercise (FE) has been shown to
provide therapeutic benefits for PD motor symptoms similar to that of
antiparkinson medication1.
In the current study, both voluntary exercise (VE) and FE were evaluated. Our
results suggest that both modes of aerobic exercise have effects on motor
functional connectivity similar to changes associated with antiparkinson
medication.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurological disorder which produces a general poverty of movement. Lower extremity forced exercise (FE) has been shown to provide therapeutic benefits for PD motor symptoms similar to that of antiparkinson medication1 . Functional connectivity fMRI (fcMRI) results demonstrated that FE and medication produce similar changes in functional connectivity patterns in motor networks2. It is important to note that in the prior study we observed that the effect of both medication and FE on functional connectivity of the motor system was highly variable, causing increased connectivity between elements of the motor system in some PD patients, and decreased in others2. In the current study, both voluntary exercise (VE) and FE were evaluated to investigate the effect of two modes of aerobic exercise on motor functional connectivity in PD with seed-based fcMRI analysis. Our results suggest that both modes of aerobic exercise have effects on motor functional connectivity similar to changes associated with antiparkinson medication.METHODS
Twelve patients with mild to moderate PD participated under an IRB-approved protocol were randomly assigned to two cycling exercise groups: FE (age 62.2±6.8; 3 males) and VE (age 59.8±12.3; 2 males). Each participant completed 3 MRI sessions: 1) on and 2) off anti-parkinsonian medication prior to the exercise intervention (MEDs and OFF MEDs) and 3) post-exercise intervention while off antiparkinson medication (FE or VE). All patients exercised on a semi-recumbent stationary cycling. The VE group pedaled at their preferred rate, while the FE group pedaled at a rate that was 35% greater than their VE rate with the assistance of a motor that augmented pedaling motion. In each MRI session, 3 whole-brain scans were performed on a 3 tesla MRI TIM-Trio with a 12-channel receive-only head coil (Siemens AG, Erlangen, Germany), using a bite bar to minimize head motion during scanning. 1. Anatomic T1 weighted 3DMPRAGE. 2. Resting state fMRI (rs-fMRI) using a 2D gradient Echo Planar imaging (EPI) sequence with FOV=256x256, 34 4mm slices, voxel size 2x2mm2, TE/TR 29ms/3s and repetition 132. 3. Task-based fMRI (tfMRI) with block-design complex finger tapping motor activation, using the same scan parameters as the rs-fMRI scan but with 160 repetitions. For image processing, rs-fMRI data were corrected to remove physiologic noise (PESTICA3), and head motion (SLOMOCO4). tfMRI data were corrected for volumetric motion (afni 3dvolreg7). Both rs-fMRI and tfMRI data were then spatially filtered. Based on tfMRI data and boxcar reference function matching with the task paradigm, whole brain Student’s t-score maps were calculated (afni 3dDeconvolve8). Seed-based fcMRI analysis method5, 6 was used. A 9-voxel in-plane ROI was placed in the left primary motor cortex (M1 LT) based on the maximum tfMRI t-score in that region and aligned to rs-fMRI data space. Rs-fMRI data were further detrended and temporally filtered to eliminate frequencies above 0.08 Hz. The correlation of the mean ROI timeseries and each voxel of the brain was calculated and normalized. Functional connectivity was calculated by averaging the 9 in-plane voxels with highest z-scores in each of the following motor network areas: right M1 (M1 RT), supplementary motor area (SMA), right/left thalamus (THAL RT/LT) and right/left putamen (PUT RT/LT). Based on our previous observation of variable changes in fcFMRI in the motor system under either medication or exercise, we investigated the change in fcMRI. For each motor area, fcMRI change was calculated by subtracting fcMRI OFF MEDs from fcMRI MEDs or fcMRI EXE, which was done for both FE and VE groups. The scatterplots and linear correlation between fcMRI change due to medication and due to exercise FE or VE were created for all motor regions listed above. P value was calculated using two-tailed Pearson method.RESULTS
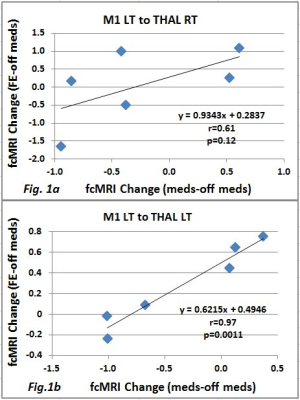
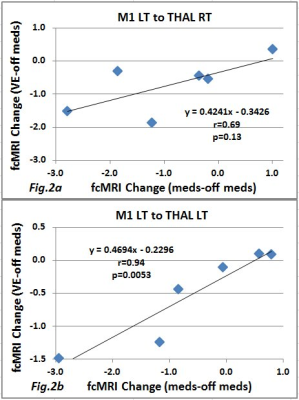
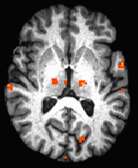
Figure 1 shows scatterplots and linear correlation of fcMRI change due to the medication and FE on THAL RT (a) with p<0.12 and THAL LT (b) with p<0.0011. Figure 2 shows the same plots but for VE on THAL RT (a) with p<0.13 and THAL LT (b) with p<0.0053. For both FE and VE, no significant difference was found for the areas M1 RT, SMA PUT RT, and PUT LT. Figure 3 shows an example of a slice with Z score map of VE.DISCUSSION and CONCLUSION
We observe changes in motor system connectivity in PD patients as a result of VE and FE interventions that are similar to antiparkinson medication responses. The results of this study indicate high intensity aerobic exercise has the potential to alter neuronal pathways and provides initial evidence for the systematic use of exercise as an adjunct to traditional pharmacological interventions.Acknowledgements
This work was supported by the National Institutes of Health R01NS673717, the Farmer Foundation and Cleveland Clinic. Author gratefully acknowledges technical support by Siemens Medical Solutions.References
- Ridgel AL. Vitek JL. Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil Neural Repair. 2009;23:600–608.
- Beall EB, Lowe MJ, Alberts JL, et al. The effect of forced-exercise therapy for Parkinson's disease on motor cortex functional connectivity. Brain Connect. 2013;3(2):190-8. doi: 10.1089/brain.2012.0104. Epub 2013 Feb 25.
- Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage. 2007 Oct 1;37(4):1286-300. Epub 2007 Jul 13.
- Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014 Nov 1;101:21-34. doi: 10.1016/j.neuroimage.2014.06.038. Epub 2014 Jun 24.
- Lowe et al. (2014) Anatomic connectivity assessed using pathway radial diffusivity is related to functional connectivity in monosynaptic pathways. Brain Connectivity. 4(7):558-65.
- Lowe MJ, et al. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998 Feb;7(2):119-32.
- Cox RW, Hyde JS. ‘Software tools for analysis and visualization of fMRI data’ NMR Biomed, 10, 171-8, (1997).Douglas Ward. Deconvolution Analysis of FMRI Time Series Data. AFNI 3dDeconvolve Documentation, Medical College of Wisconsin, May 2002.
- Douglas Ward. Deconvolution Analysis of FMRI Time Series Data. AFNI 3dDeconvolve Documentation, Medical College of Wisconsin, May 2002.