1934
Baseline Symptoms and Basal Forebrain Volume Predict Future Psychosis in Early Parkinson Disease1Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States, 2Neurology, University of Virginia, Charlottesville, VA, United States, 3Public Health Services, University of Virginia, Charlottesville, VA, United States
Synopsis
Psychosis is a common neuropsychiatric symptom of Parkinson’s disease, and can serve as a clinical marker of advanced disease. Our study aimed to investigate the characteristics of psychosis in a longitudinal PD cohort, to verify baseline clinical risk factors for future psychotic symptoms in de novo PD patients, and to evaluate the relationship between baseline gray matter density in the nucleus basalis of Meynert and future psychotic symptoms in PD. We found lower NBM density at baseline to be associated with increased psychotic symptom burden compared to controls, suggesting utility for the NBM as a neuroimaging biomarker for advanced PD.
Introduction
Psychosis is a common neuropsychiatric symptom in Parkinson’s disease (PD). Psychosis in PD is associated with dementia,1 increased institutionalization,2 and increased mortality.3 In addition to being a clinical marker of advanced disease, it is also a marker of advanced alpha-synuclein pathology. Determining baseline predictors of future psychosis in PD may identify those at risk for more rapidly progressive disease, i.e. a more malignant PD subtype. Degeneration of the nucleus basalis of Meynert (NBM), which provides cholinergic innervation to the entire neocortex, is a feature of PD and PD dementia.4,5 We aimed to determine the relationship between NBM degeneration and future psychosis in PD.Methods
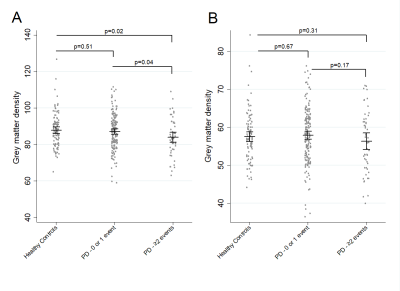
This study evaluated 423 newly diagnosed PD subjects collected as part of the Parkinson’s Progression Markers Initiative. PD symptoms were assessed using the Movement Disorders Society – Unified Parkinson Disease Rating Scale item 1.2, which assesses hallucinations and psychosis over the past week. At baseline, subjects completed the Scales for Outcomes in Parkinson's disease – Autonomic (SCOPA-AUT), the REM Sleep Behavior Disorder (RBD) Screening Questionnaire, and the Epworth Sleepiness Scale. Grey matter density was measured using voxel based morphometry methodology6,7 applied to MP-RAGE T1 images collected at baseline for 226 PD subjects and 99 healthy controls. NBM density was measured according to its location within cholinergic nuclei 4 (Ch4), with cholinergic nuclei 1-3 serving as a control. Ch4 and Ch1-3 nuclei were separately measured using previously published probabilistic masks.8Result
Multivariate logistic regression adjusted for age and sex found that greater autonomic symptoms (p=0.002), RBD (p=0.021), and excessive daytime sleepiness (EDS) (p=0.003) at baseline were associated with increased risk of reporting psychotic symptoms on 2 or more occasions. Having 2 or 3 of these baseline symptoms was significantly associated with lower Ch4 density (p=0.007) but not with Ch1-3 density (p=0.45). In a logistic regression model adjusted for age and sex, higher Ch4 density was associated with lower risk of reporting psychotic symptoms on 2 or more occasions (OR=0.96 (for an increase in density of 1 unit), p=0.03).Discussion
Even without accounting for the effect of medications, we found that a triad of clinical symptoms and lower Ch4 density were associated with future psychotic symptoms. There is no direct evidence that lower Ch4 density has a causal relationship with these 3 non-motor symptoms. Instead, these symptoms and Ch4 density are likely both associated with more widespread but not ubiquitous subcortical pathology. This idea is supported by the association between the triad of non-motor symptoms and Ch4 density but not Ch1-3 density. Additionally, the role of Ch4, but not Ch1-3, as a primary source of cholinergic input to the neocortex further underscores the importance of studying the role of cholinergic pathways in the development of non-motor symptoms in PD.Conclusion
This study confirms that RBD, EDS, and greater autonomic symptom burden are associated with greater risk of future psychotic symptoms in PD. Reduced Ch4 density at baseline is associated with future psychotic symptoms and a greater burden of RBD, EDS, and autonomic symptoms. The relationship between this triad of clinical symptoms and lower Ch4 density support the potential utility of this neuroimaging biomarker to identify a diffuse malignant subtype of PD and to predict more rapid disease progression.Acknowledgements
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI- a public-private partnership- is funded by the Michael J. Fox Foundation (MJFF) for Parkinson’s Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen, Britsol-Myers Squibb, Covance, GE Healthcare, Genetech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier, and UCB. The MJFF was not involved in the data analysis for this article. Neither the funding agency nor any of the sponsors of the PPMI were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.References
1. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in parkinson disease: An 8-year prospective study. Arch Neurol 2003;60:387-392.
2. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in parkinson's disease: A population-based, prospective study. J Am Geriatr Soc 2000;48:938-942.
3. Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced parkinson's disease. Neurology 1995;45:669-671.
4. Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, Tomlinson BE. Pathological changes in the nucleus of meynert in alzheimer's and parkinson's diseases. J Neurol Sci 1983;59:277-289.
5. Perry EK, Curtis M, Dick DJ, et al. Cholinergic correlates of cognitive impairment in parkinson's disease: Comparisons with alzheimer's disease. J Neurol Neurosurg Psychiatry 1985;48:413-421.
6. Mechell A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev 2005;1:105-113.
7. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage 2000;11:805-821.
8. Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 2008;42:1127-1141.
Figures