1925
The longitudinal changes in white matter of patients with Parkinson's disease as detected by using Fixel-Based Analysis1Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan, 2Neurology, Chang Gung Memorial Hospital, Taoyuan, Taiwan, 3Diagnostic Radiology, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan, 4Diagnostic Radiology, Chang Gung Memorial Hospital, Taoyuan, Taiwan, 5Division of Imaging Sciences & Biomedical Engineering, King's College London, London, United Kingdom
Synopsis
Parkinson's disease (PD) is a neurodegenerative disease as the result from the loss of cell in basal ganglia. Fixel-Based Analysis can qualified the fiber density and fibre-bundle cross-section in the white matter. The fiber density and fibre-bundle cross-section is feasible to interpret the microstructure changes in the brain of patients with PD. Therefore, the current study aimed to investigate the long-term white matter changes in Parkinson's disease by using Fixel-Based Analysis.
Introduction
Parkinson's disease (PD) is a neurodegenerative disease as a result from the loss of dopaminergic cell in basal ganglia. Because of the nature of slow progression, the microstructural changes in the brain of patients during the disease course is of great interest. Fixel-Based Analysis is a new approach that can qualify the fiber density and fibre-bundle cross-section in the nerve fibers. Therefore, the current study proposed to investigate the changes in white matter in the patients with Parkinson's disease over a period of 4 years.Methods
Multi-shell diffusion weighted images with the whole brain coverage were acquired from 48 PD patients (aged 64.7 ± 6.5 year old) using 3T scanner (Trio, Magnetom, Siemens, Erlangen Germany) in the baseline and the fourth year (after 258.8 ± 26.0 weeks). The imaging parameters included TR/TE = 5700 ms/108 ms; voxel size=2×2×2 mm, b=0, 1000, 2000, 3000 s/mm2; 30 directions per shell. Fixel-Based Analysis was performed using MRtrix3 following the recommended pipeline (1) , including MP-PCA denoising (2), Gibbs ringing removal (3), motion and distortion correction (4,5), and bias field correction (6). The distribution of fiber orientations within each imaging voxel was estimated using multi-tissue constrained spherical deconvolution (7). A study-specific template was then created by spatial normalization over all subjects using FOD-based registration (8). Within each voxel, fixel-specific measures of both fiber density (FD) and fibre bundle cross-section (FC) were calculated. To obtain a more complete measure of white matter changes, a combined measure was also estimated by a simple multiplication of the fiber density and the fibre-bundle cross-section (FDC) (8). White matter changes over the follow-up period was determined using voxelwise paired t-tests, with significance (p = 0.05) determined using connectivity-based fixel enhancement (9) to correct for multiple comparison.Result and Discussion
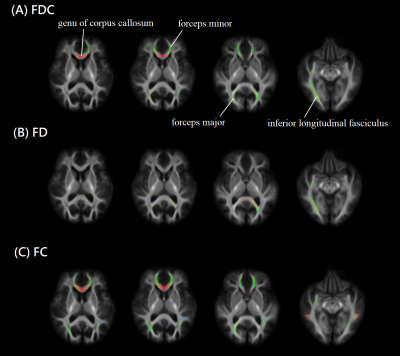
Figure 1 showed the result from fixel-based analysis. Changes in fiber density & bundle cross-section during the follow-up period was overlaid on the study-specific template, noticeably in anterior forceps, posterior forceps, inferior longitudinal fasciculus and genu of corpus callosum. Such changes can be attributed to a decrease in the fiber density, for example, posterior forceps and inferior longitudinal fasciculus. Alternatively, it can be related to a decrease in the fibre-bundle cross-section, such as those located in anterior forceps, posterior forceps and genu of corpus callosum. Previous studies showed that the decreased Fractional Anisotropy and increased Mean Diffusivity can be found in the skeleton of corpus callosum or the forceps in patients of PD with either cognitive impairment (10) or depression (11). Our study identified the affected regions in patients with PD over a 3 years follow-up period. The Fixel analysis detected the changes in white matter with improved spatial information rather than skeleton, which can be attributed to either a decrease in the neuron fiber density or the fiber bundle cross-section, or both.Conclusion
Over a follow-up period for four years, regions with significant reductions in white matter density or cross-section area can be identified in the corpus callosum and its neighborhood. These changes observed using a longitudinal analysis match observations from other studies that used cross-sectional analysis. Fixel-based analysis detected longitudinal changes in whiter matter from patients with Parkinson's disease over a 3 year period.Acknowledgements
Fibre-tracking was performed using the MRtrix package (J-D Tournier, Brain Research Institute, Melbourne, Australia, https://github.com/MRtrix3/mrtrix3) (Tournier et al. 2012)References
1. Raffelt DA, Tournier J-D, Smith RE, Vaughan DN, Jackson G, Ridgway GR, et al. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage 2016.
2. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage 2016;142:394–406.
3. Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2015.
4. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016;125:1063–1078.
5. Andersson JLR, Sotiropoulos SN. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. NeuroImage 2015;122:166–176.
6. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010;29:1310–1320.
7. Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 2014;103:411–426.
8. Raffelt D, Tournier J-D, Fripp J, Crozier S, Connelly A, Salvado O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 2011;56:1171–1180.
9. Raffelt DA, Smith RE, Ridgway GR, Tournier J-D, Vaughan DN, Rose S, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. NeuroImage 2015;117:40–55.
10. Goldman JG, Bledsoe IO, Merkitch D, et al. Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease. Neurology. 2017 Mar 28;88(13):1265-1272.
11. Peiyu Huanga, Xiaojun Xua, Quanquan Gu, et al. Disrupted white matter integrity in depressed versus non-depressed Parkinson's disease patients: A tract-based spatial statistics study. J Neurol Sci. 2014 Nov 15;346(1-2).
Figures