1924
Determination of White Matter Tracts Implicated in Postural Gait Instability Disorder through Tract-Based Automated Analysis1Department of Research, National Neuroscience Institute, Singapore, Singapore, 2Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan, 3Health Services Research Unit, Singapore General Hospital, Singapore, Singapore, 4Department of Diagnostic Radiology, Singapore General Hospital, Singapore, Singapore, 5Duke-NUS Medical School, Singapore, Singapore, 6Department of Neurology, Singapore General Hospital, Singapore, Singapore, 7Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
Tract-Based Automated Analysis (TBAA) in Diffusion Tensor Imaging allows for the study of microstructural properties along the tracts in white matter. Diffusivity measures extracted from TBAA for various tracts of the brain were correlated to Tinetti Balance Scale scores in Parkinson's Disease and Postural Gait Instability Disorder patients, allowing identification of tracts of interest in the pathological study of the diseases.
Introduction
Patients diagnosed with Postural Gait Instability Disorder (PGID) and Parkinson’s Disease (PD) have been shown to have microstructural alterations in their white matter (WM) tracts[1],[2]. The alterations in the nigrostriatal pathway in particular, are believed to account for the varied motor and non-motor features in the subtypes of PD[3]. Diffusion Tensor Imaging (DTI) in Magnetic Resonance Imaging (MRI) provides a way of studying these alterations. Tract Based Automated Analysis (TBAA) is an automated method of tractography using diffusion MRI which allows reliable sampling of microstructural properties along tracts, while overcoming the limitations of voxel-based methods[4]. The study of diffusivity measures taken along neural tracts give an indication of the microstructural differences between the formerly mentioned study groups, and are able to assist in their differentiation[5]. This study assesses the ability of the indices generated through TBAA to identify group differences in tracts between PGID, PD and Healthy Controls (HC) groups, and attendant clinical implications.Methods
66 subjects [25 PGID, 21 PD and 20 age-matched HC] underwent brain 3T MRI, including DTI (spin echo echo-planar; 30 directions; b values 0, 800 s/mm2; TE/TR 86/8200 ms; voxel size 1.9×1.9x2mm3). 31 WM tracts which are hypothesized to be associated with PGID and PD were selected for analysis. The DTI images were pre-processed and corrected for WM lesions to reduce registration errors prior to TBAA. Subsequently, the images were normalised using connectivity maps and converged to form a Study Specific Template, and registered to a high-quality Diffusion Spectrum Imaging template[4]. The indices from each tract were extracted using the atlas from TBAA, resulting in the following: fractional anisotropy (FA), mean, axial and radial diffusivity (MD, AD and RD). A univariable regression was performed for theses indices (averaged for paired tracts), with Tinetti Balance Scale (TBS) scores (clinical assessment of stability in gait). Tracts showing indices with a correlation to each study group were examined for functionality type.Results
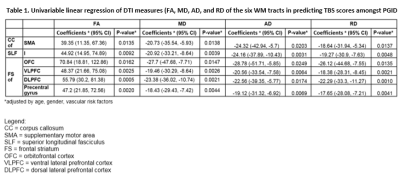
There were significant (p < .017, Bonferroni corrected) DTI abnormalities for multiple WM tracts in PGID compared to both PD and HC by means of Mann-Whitney U tests, and differences more marked between PGID and HC. Conversely, no WM tract difference was seen between PD and HC. Univariable regression on TBS scores in PGID correlated (p < .025) with changes in all DTI measures for 6 WM tracts (CC of SMA, SLF I, FS of OFC, VLPFC, DLPFC; Table 1).Discussion
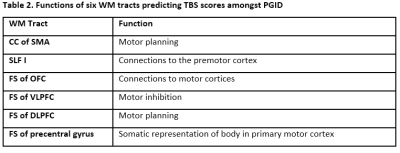
The 6 WM tracts showing DTI abnormalities in the PGID group have been related to motor abilities (described in Table 2)[6],[7],[8]. The results of linear regression show that a patient who is more unstable (with a lower TBS score) would typically have decreased FA and increased MD, AD and RD values. This shows a loss in directionality of diffusion throughout the tract as it degrades.
TBAA provides a comprehensive atlas, opening a pathway to study the pathophysiology of neurodegenerative diseases such as PD and PGID. There are a plethora of WM tracts with plausible relations to motor function. However, the use of DTI, paired with automation methods in tractography, provides a possible quantitative method of identifying the severity of the disease, as well as the ability to pinpoint the tracts that show relation to this ailment.
Conclusion
TBAA rapidly and objectively characterises whole brain WM circuitry, demonstrating widespread changes in the commissural, projection and association tracts in PGID compared to PD. These abnormal WM tracts could serve as potential biomarkers for PGID and its disease progression, furthering our understanding of the underlying pathophysiology.Acknowledgements
We would like to thank the National Medical Research Council, Singapore, and Siemens, Singapore, for their support.References
[1] Canu E, Agosta F, Markovic V, Petrovic I, Stankovic I, Imperiale F, Stojkovic T, Copetti M, Kostic VS, Fillipi M. White matter tract alterations in Parkin’s disease patients with punding. Parkinsonism and Related Disorders 2017; 43: 85-91
[2] Tan S, Keong N, Thien A, Li HH, Rumpel H, Tan EK, Chan LL. Periventricular Longitudinal Neural Tracts Are Implicated in Postural Instability Gait Disorder. ISMRM 2016; 6620
[3] Alexander GE, Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clinical Neuroscience 2004; 6(3):259-80
[4] Chen YJ, Lo YC, Hsu YC, Fan CC, Hwang TJ, Liu CM, Chien YL, Hsieh MH, Liu CC, Hwu HG, Tseng WY. Automatic whole brain tract-based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Human Brain Mapping. 2015 Sep;36(9):3441-58.
[5] Chan LL, Ng KM, Rumpel H, Fook-Chong S, Li HH, Tan EK. Transcallosal diffusion tensor abnormalities in predominant gait disorder Parkinsonism. Parkinsonism and Related Disorders. 2014 Jan;20(1):53-9.
[6] Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing Superior Longitudinal Fasciculus Connectivity in the Human Brain using High Resolution Diffusion Tensor Tractography. Brain Structure Function. 2014 Jan; 219(1)
[7] Levy BJ, Wagner AD. Cognitive control and right ventrolateral perfrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011 Apr; 1224(1):40-62.
[8] Zgaljardic DJ, Mattis PJ, Charness A. Executive Dysfunction. Encyclopedia of Movement Disorders. 2010; 458-62