1923
Cerebral blood flow in a resuscitated septic shock population: an ASL study1Université de Sherbrooke, Sherbrooke, QC, Canada, 2Centre de recherche du CHUS, Sherbrooke, QC, Canada, 3Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4Mount Sinai Hospital, Toronto, ON, Canada
Synopsis
Reduced cerebral blood flow (CBF) is often blamed for sepsis-associated encephalopathy. The present study compares the CBF and blood oxygen consumption (CMRO2) of healthy subjects and resuscitated septic patients under vasopressor (norepinephrine) treatment. Methods used are pseudo-continuous arterial spin labeling (PCASL) and T2-relaxation-under-spin-tagging (TRUST). We find that septic patients have elevated global and regional CBF, whereas CMRO2 seems reduced. Further studies are needed to elucidate the underlying mechanisms of this apparent uncoupling.
Introduction
Septic shock is a life-threatening condition characterized by a significant drop in systemic blood pressure. Sepsis is often accompanied by brain dysfunction1. The underlying mechanisms of this sepsis-associated encephalopathy remain unclear, but evidence suggests that reduced cerebral blood flow (CBF) plays a major role.2,3
This study compares the cerebral blood flow (CBF) of resuscitated septic shock patients to that of a normal population using pseudo-continuous arterial spin labeling (PCASL).
Methods
Healthy volunteers (n = 12) with (n = 6) and without (n = 6) controlled hypertension were compared to septic patients (n = 10; 4 with diagnosed hypertension) under propofol sedation, mechanical ventilation and vasopressor (norepinephrine) treatment.
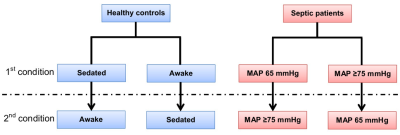
Each subject underwent two consecutive CBF measurements using 2D PCASL on a 3T Philips Ingenia with acquisition parameters following recommendations by Alsop et al.4 Imaging conditions were randomly assigned (Figure 1). Venous oxygenation was measured using the TRUST method in septic patients.5,6
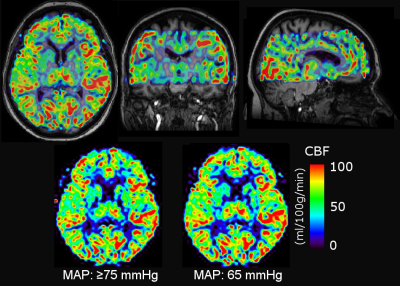
Quantitative CBF maps were produced using FSL (Figure 2). Registration with the T1‑weighted anatomic image as well as segmentation and partial volume correction were performed in PMOD. Venous oxygenation was derived in MATLAB using functions provided by Dr Peiying Liu (pliu27@jhmi.edu). The cerebral metabolic rate of oxygen (CMRO2) was calculated using image-derived venous oxygenation as well as blood oxygen saturation and hematocrit from standard methods. Statistical analysis was performed in GraphPad Prism. Significance level was set at p ≤ 0.05 with Bonferroni correction for multiple comparisons.
Results
One healthy subject was excluded due to excessive motion, which prevented CBF calculation. One septic patient was also excluded upon observing severe reduction of blood flow in a hemisphere following a stroke.
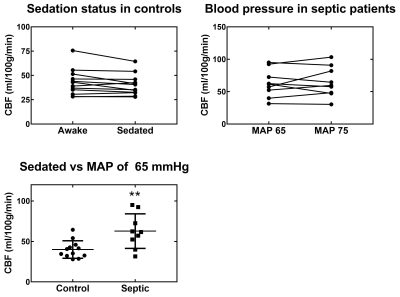
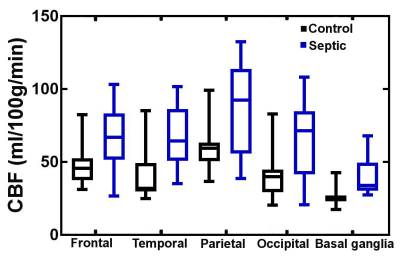
No significant differences were observed between non-hypertensive and hypertensive sub-groups, so their data were pooled in the analysis. Figure 3 summarizes our main findings for global CBF. There is a non-significant (p = 0.06) trend towards decreased CBF (-7 %) in sedated healthy controls. There is no significant CBF change between blood pressure targets in septic patients. Interestingly, the global CBF for septic patients is 37-47% higher (depending on MAP target and sedation status) than for healthy controls (p < 0.01). The same trend was observed regionally (Figure 4).
No significant difference is seen for CMRO2 in septic patients between MAP targets (respectively 84 ± 14 and 77 ± 19 μmol/100g/min at 65 and ≥75 mmHg). However, these values are inferior to those reported for healthy subjects (~130 μmol/100g/min). 7
Discussion
Our methodology now includes region-based analysis as well as partial volume correction (to account for cortical atrophy in older subjects). Results are consistent with our previous report on global measures8.
The higher CBF in many septic patients compared to healthy controls is both striking and unexpected. Previous research indicated that brain perfusion decreases in septic patients9,10. Moreover, animal models of resuscitated septic shock showed evidence of neuronal ischemia11. Many factors may influence the CBF of septic patients, for example: time elapsed after shock, severity of shock, choice of vasopressor and sedation practices. All of these need to be assessed in a larger cohort to confirm and explain the present results. It is also interesting that this increase in global CBF is reflected in the basal ganglia, a region thought to be involved with sepsis-induced delirium12.
Furthermore, blood oxygen consumption appears to be reduced in septic patients despite the increased CBF. Uncoupling of blood flow and oxygen consumption is thought to occur in sepsis13,14, and a similar pattern of increased blood flow with normal/decreased oxygen consumption has been reported in the kidneys of septic sheep.15 This also warrants further studies.
Limitations
Cohorts were small and it is not surprising that the trend for decreased CBF under propofol sedation was not statistically significant even though this is a documented effect16. For the same reason, we cannot draw conclusions regarding the effect of MAP in septic patients. We have also considered possible pitfalls of the ASL method, such as the impact of blood transit time and differences between the subject cohorts (e.g. older age of septic patients), but all these effects would lead to a decrease rather than an increase in CBF. So far, we deem that our MR-based method is reliable.Conclusion
We have observed an unexpected increase in CBF in several resuscitated septic patients compared to healthy controls. The impact of treatment protocols on the apparent hyperperfusion and its implications for septic encephalopathy call for further investigation.Acknowledgements
The authors wish to thank Guillaume Gilbert for his expertise in implementing the MR protocol and the team of MR technologists at the CHUS for their outstanding work.References
1. Pytel P and Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol. 2009;22(3):283-287.
2. Burkhart CS, Siegemund M and Steiner LA. Cerebral perfusion in sepsis. Crit Care. 2010;14(2):215.
3. Stocchetti N, et al. Neuroprotection in acute brain injury: an up-to-date review. Crit Care. 2015;19(1):186.
4. Alsop DC, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102-116.
5. Lu H and Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60(2):357-363.
6. Xu F, Ge Y and Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med. 2009;62(1):141‑148.
7. Jain, V, Langham MC and Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30(9): 1598-1607.
8. Lamontagne F, et al. Arterial spin labeling magnetic resonance imaging evaluation of cerebral perfusion in health and under vasopressor therapy for sepsis. 30th Annual Congress of the European Society of Intensive Care Medicine. 23-27 September 2017, Vienna, Austria.
9. Bowton DL, et al. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit Care Med. 1989;17(5): 399-403.
10. Maekawa T, et al. Cerebral circulation and metabolism in patients with septic encephalopathy. Am J Emerg Med. 1991;9(2):139-143.
11. Taccone FS, et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med. 2014;42(2):e114-22.
12. Pfister D, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008; 12(3):R63.
13. Miranda M, et al. Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circ Physiol. 2016;311(1):H24-35.
14. Rosengarten B., et al. Early neurovascular uncoupling in the brain during community acquired pneumonia. Crit Care. 2012;16(2): R64.
15. Calzavacca P, May CN and Bellomo R. Glomerular haemodynamics, the renal sympathetic nervous system and sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2014;29(12):2178-2184
16. Kaisti KK, et al. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96(6):1358-1370.
Figures