1915
Noninvasive measurements of human brain temperature in patients with arteriovenous malformations using magnetic resonance spectroscopy1Neurosurgery, Sendai Medical Center, Sendai, Japan, 2Neurosurgery, Kohnan Hospital, Sendai, Japan, 3Neurosurgery, Tohoku University, Sendai, Japan
Synopsis
The present study investigated whether brain temperature measured by proton magnetic resonance (MR) spectroscopy can detect cerebral hemodynamic impairment in patients with arteriovenous malformations (AVMs) as shown by single photon emission computed tomography (SPECT). Brain temperature, cerebral blood flow, and cerebrovascular reactivity were measured using proton MR spectroscopy and SPECT in five healthy volunteers and six patients with AVMs. A significant correlation was observed between brain temperature difference (affected side - contralateral side) and cerebrovascular reactivity ratio (affected side/contralateral side) (r=0.82, p=0.0480). Brain temperature measured by proton MR spectroscopy can detect cerebral hemodynamic impairment in patients with AVMs.
Background and Purpose
In clinical practice, local temperature measurements require the insertion of a specific probe. This procedure is appropriate, but noninvasive measurement is desirable in neurosurgical practice. The brain temperature at rest is determined by the balance between heat produced by cerebral energy turnover, which is identical to cerebral metabolism, and heat that is removed, primarily by cerebral blood flow. Recent advances in magnetic resonance (MR) imaging technology allow measurement of brain temperature using MR spectroscopy (1,2,3). The present study investigated whether brain temperature measured by proton MR spectroscopy can detect cerebral hemodynamic impairment in patients with arteriovenous malformations (AVMs) as shown by single photon emission computed tomography (SPECT).Patients and Methods
This study included five
healthy volunteers and six patients with AVMs, three females and three males
aged 17-50 years, who underwent MR imaging and CBF dynamics study within 5
days. Brain temperature, cerebral
blood flow, and cerebrovascular reactivity were measured using proton MR
spectroscopy and SPECT. All MR imaging used a 3.0 Tesla MR imaging system
and parallel imaging head coil. T2-weighted MR imaging used the short inversion
time inversion recovery sequence with the following parameters: repetition time
(TR) 4000 ms, echo time (TE) 25 ms, inversion time 100 ms, matrix 512 x 384,
field of view (FOV) 240 mm, and 3.5 mm slice thickness. Single voxel MR
spectroscopy was performed with the following parameters: TR 2000 ms, TE 136
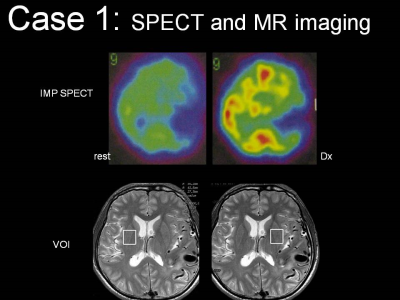
ms, and 128 excitations. The voxel of interest (VOI) was set in the normal
brain tissue adjacent to the AVM lesion identified on the T2-weighted image,
and in the corresponding location in the contralateral hemisphere (Fig. 1 Lower
row). The VOI size was 20 x 20 x 30 mm. The MR spectrum showed
choline-containing compounds at 3.2 ppm, creatine phosphate at 3.0 ppm, and
N-acetyl aspartate (NAA) at 2.0 ppm. The brain temperature was calculated from
the chemical shift between NAA and water using original software. The ratio of
the value in the affected hemisphere to that in the contralateral hemisphere
was then calculated in each patient. While obtaining MR spectroscopy, the environment
temperature was maintained at 21-25 ˚C. Regions of interest were
selected adjacent to the AVMs and in the corresponding contralateral region
(Fig 1.).Results
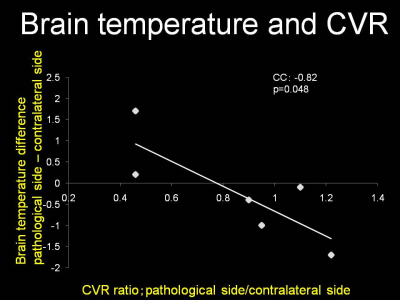
The mean brain temperature measured by MR spectroscopy in volunteers was 37.1 ± 0.41 ˚C. The mean brain temperature measured by MR spectroscopy in patients with AVMs was 37.8 ± 1.1 ˚C in the pathological side and 38.0 ± 0.9 ˚C in the contralateral side. Figure 2 shows the brain temperature ratio measured by MR spectroscopy and CVR ratio measured by SPECT in each patient. The fit to the regression line of the values obtained in the subjects was significant (p = 0.0480), with a correlation coefficient of -0.82. There was no significant correlation between AVM size and brain temperature ratio.Discussion
The present study suggested that MR spectroscopy could measure the brain temperature around AVMs, and that the brain temperature was correlated with the CVR measured by SPECT. Positron emission tomography has shown that the cerebral metabolic ratio of O2 around AVMs does not differ from the contralateral region, and the oxygen extraction fraction (OEF) is significantly increased in patients with AVMs. Brain temperature is reported to be correlated with cerebra blood volume and OEF in patients with chronic major cerebral artery occlusive disease. Our present finding of a significant negative correlation between brain temperature and CVR suggests that the balance between cerebral perfusion and cerebral metabolism determines the brain temperature around AVMs, and reduced cerebral perfusion relative to cerebral metabolism results in decreased removal of the heat produced by cerebral energy turnover, resulting in increased brain temperature. Massive multifocal bleeding after technically successful removal of an AVM represents a frightening and usually catastrophic complication. This phenomenon, termed normal perfusion pressure breakthrough, is caused by the diversion of blood flow from the AVM into adjacent, maximally dilated, and nonautoregulating small vessels. If normal perfusion pressure breakthrough occurs during an operation, the surgeon will be unable to control the resultant hemorrhage by standard microsurgical techniques. Patients with reduced CVR adjacent to the AVM tend to suffer normal perfusion pressure breakthrough. Therefore, estimating the brain temperature around AVMs may offer a noninvasive method for the preoperative identification of patients with AVM at risk for normal perfusion pressure breakthrough.Conclusion
Brain temperature measured by proton MR spectroscopy can detect cerebral hemodynamic impairment in patients with AVMs. Further investigations regarding the relationships between brain temperature and risk of surgery in patients with AVMs are needed (4).Acknowledgements
This work was supported by JSPS KAKENHI Grant Number24592112.References
1. Cady EB et al. MRM 1995
2.Yoshioka Y, Inoue T et al.J App Physiology 2005
3.Ishigaki D, Inoue T et al. Stroke 2009
4. Inoue T et. al. CNN 2013
Figures