1902
Comparison of Cerebral Blood Flow in a Rat Model of Hypertension and Age-Matched Controls1Laboratory of Clinical Investigation, Magnetic Resonance Imaging and Spectroscopy Section, National Institute on Aging, Baltimore, MD, United States, 2Laboratory of Cardiovascular Sciences, National Institute on Aging, Baltimore, MD, United States
Synopsis
Continuous arterial spin labeling (CASL) was used to quantify and compare cerebral blood flow (CBF) in Dahl salt-sensitive (DSS) and Sprague-Dawley (SD) rats. CBF quantification was greatly facilitated through use of the recently-introduced NESMA non-local noise reduction filter. A blunted response to hypercapnia was observed in the DSS rats. These results demonstrate the dysregulation of cerebral vasodilatory responses in hypertension, and may have important implications in the understanding of the vascular basis for cognitive impairment in humans.
Introduction
The Dahl salt-sensitive (DSS) rat is an important model of salt-sensitive hypertension (HTN).1-3 While these rats will develop HTN even in the absence of high salt diets, the progression of HTN can be titrated by altering their sodium consumption.2,3 DSS rats fed low-to-normal salt diets have been found to have reduced levels of angiotensin II, which can contribute to impaired vasodilation and endothelial dysfunction.4 In addition, there is increasing recognition that such vascular and cerebral blood flow (CBF) abnormalities may be causally linked to the development of mild cognitive impairment and Alzheimer’s disease.5 We therefore sought to evaluate the cerebral vascular reactivity of the DSS HTN model using continuous arterial spin labeling (CASL) MRI. We hypothesized that the DSS rats would demonstrate hypoperfusion even at an early age and blunted hemodynamic response to increasing CO2 concentration due to impaired vasodilatory capacity.Materials and Methods
Image Acquisition
Sprague-Dawley (SD; n=3) and DSS (n=5) rats, (male, 6 months of age) receiving a normal salt (0.5% NaCl) diet were used for this study. Systolic blood pressure (SBP) was measured using tail cuff plethysmography at age 3 and 6 months. Rats were scanned with a single-slice CASL sequence with single-shot EPI acquisition at age 6 months. Vascular reactivity was evaluated by measuring CBF under air (~0.04% CO2) and 5% CO2/95% inhalation, with increased CO2 serving as a vasodilatory stimulus. Scans were performed on a 7T Bruker system using an 86-mm quad coil with a 2 x 2 four-element rat head array coil. Rats were anesthetized with 3 L/min oxygen and 2% isoflurane and maintained at 1 L/min oxygen and 1.5-2.0% isoflurane and at 37°C, with monitoring of vital signs (SA Instruments, New York). Respiration rate was maintained at 60-90 breaths/min. Data were acquired with TR = 10s, labeling duration = 2s, post-labeling delay (PLD) = 0.1s, in-plane spatial resolution = 0.234 X 0.234 mm2, FOV = 30 x 30 mm2, and signal averages = 15 on a single 1.0 mm-thick axial slice placed at the largest hippocampal extent. Control (SIC), labeled (SIL), and proton density (SIPD) images were acquired for each inhalation condition.
Cerebral Blood Flow Mapping
We used the following expression for CBF determination6
$$CBF = \frac{6000 \cdot \lambda\cdot (SI_c - SI_L)\cdot e^{\frac{PLD}{T_{1blood}}}}{2\cdot T_{1blood}\cdot SI_{pd}\cdot(1-e^{-(\frac{\tau}{T_{1blood}})})} ml/100g/min$$
where λ = 0.9 ml/g is taken as the blood brain partition coefficient, τ = 2 seconds and T1Blood = 2.4 seconds was assumed.7,8 We implemented the recently introduced NESMA filter to improve SNR to accurately measure differences in signal intensity due to perfusion.9-11
Results and Discussion
Figure 1 displays the age-associated differences in SBP between the DSS and SD rats. A 2-way ANOVA showed that the DSS rats exhibited significantly higher SBP compared to their SD counterparts at 3 and 6 months of age (p < 0.05).
Figure 2 shows the improvement in CBF maps through application of the NESMA filter. The NESMA-filtered images displayed higher resolution and less random variation. The filters thus allowed for accurate data analysis between CBF maps. Figure 2 also compares the CBF maps derived for representative SD and DSS rats. Visual inspection shows clear regional differences in CBF between the SD and the DSS rats. Overall, SD rats displayed higher perfusion compared to their hypertensive DSS counterparts. In addition, SD rats displayed an increase in CBF upon inhalation of CO2.
Figure 3 displays the quantitative CBF values obtained from the whole brain and cerebral cortex in DSS and SD rats under two different inhalation conditions. A paired T-test found that whole brain CBF values were significantly greater for 5% CO2 inhalation (110.10 ± 15.24 ml/100g/min) compared to air (69.16 ± 11.93 ml/100g/min) in the SD rat group (p = 0.040). This increase between CO2 (135.08 ± 23.76 ml/100g/min) and air (93.75 ± 8.62 ml/100g/min) was also found in the cerebral cortex (p = 0.006). In contrast, the DSS rats showed a blunted response to increased CO2, indicating dysregulation of vascular reactivity. This observation is supported by literature indicating that hypoperfusion may result from abnormalities in the neurocardiovascular loop secondary to HTN.12 The dysregulation of the cerebral vascular response due to chronic HTN may have important implications for the development of cognitive impairment and dementia, in which perfusion abnormalities, and more specifically hypoperfusion, are increasingly implicated.12
Conclusions
CBF response to increased CO2 was blunted in DSS rats as compared to SD controls. This is consistent with dysregulation of cerebral vasodilatory responses secondary to HTN. Furthermore, an ongoing longitudinal study in our lab will allow for evaluation of aging effects.Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.References
1. Dahl LK, Knudsen KD, Heine MA, Leitl GJ. Effects of chronic excess salt ingestion. Modification of experimental hypertension in the rat by variations in the diet. Circ Res 1968;22:11–18.
2. Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 1998;39: 89–105.
3. Rapp JP. Dahl salt-susceptible and salt-resistant rats. Hypertension 1982 ;4 :753–763.
4. Peric D, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension 2005 ;45 :687-691.
5. Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol 2000;7:93-100.
6. Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015 ;73:102-116.
7. Herscovitch P, Raichle ME. What is the correct value for the brain-blood partition coefficient for water? J Cereb Blood Flow Metab 1985;5:65–69.
8. Barbier EL, St Lawrence KS, Grillon E, Koretsky AP, Decorps M. A model of blood-brain barrier permeability to water: accounting for blood inflow and longitudinal relaxation effects. Magn Reson Med. 2002;47(6):1100-1109.
9. Bouhrara M, Bonny JM, Ashinsky BG, Maring MC, Spencer RG. Noise estimation and reduction in magnetic resonance imaging using a new multispectral nonlocal maximum-likelihood filter. IEEE Trans Med Imag 2017 ; 36 :181-93.
10. Maring MC, Bouhrara M, Spencer RG. A Fast Adaptive Multispectral nonlocal denoising filter. Proc ISMRM 2017.
11. Bouhrara M, Maring MC, Reiter DA, Bonny JM, Spencer RG. Enhanced quality of myelin water fraction mapping from GRASE imaging data of human brain using a new nonlocal estimation of multi-spectral magnitudes (NESMA) filter. Proc ISMRM 2017.
12. De La Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012 ; 367516
Figures
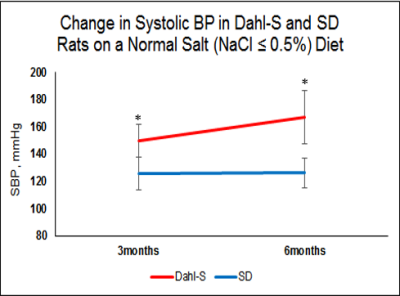
Figure 1: Systolic blood pressure in DSS (N=5) and SD (N=3) rats provided with a normal salt diet. There were significant differences in blood pressure between groups at 3 months of age, with this difference increasing with increasing age. Data is presented as mean ± standard deviation.
*P<0.05 SD vs. DSS by 2-way ANOVA
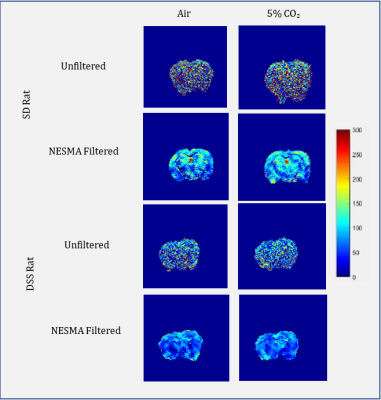

Figure 3: Means and standard deviations of CBF values obtained from the whole brain and cerebral cortex of DSS (N=5) and SD (N=3) rats for different inhalation mixtures, with increased CO2 serving as a vasodilatory stimulus. A significant increase in CBF in response to hypercapnia was observed for SD but not DSS rats. Data is presented as mean ± standard deviation.
*P <0.05 by paired T-test