1893
Visualizing the Lenticulostriate Arteries at 3T with a Dual-Echo White-Blood and Black-Blood Imaging Technique1Radiology and Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 2Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB, Canada
Synopsis
A dual-echo white-blood (WB) and black-blood (BB) imaging technique was developed to visualize the lenticulostriate arteries at 3T. The WB echo, effectively a flow-compensated time-of-flight image, and the flow-sensitized BB echo complement each other such that using these two inherently co-registered echoes in unison helps to better depict and delineate the vessels.
Purpose
Vascular dementia and cognitive impairment (e.g., Alzheimer’s disease, Lewy body dementia, frontal and temporal lobe dementia) are caused by problems in the supply of blood to the brain, most typically resulting from a multiplicity of transient ischemic attacks (TIAs) or minor strokes.1 TIAs in small vessels such as the lenticulostriate arteries (LSAs) are particularly insidious as LSA impairment often leads to lacunar infarcts and cerebral hemorrhage.
Visualizing the LSAs can be challenging as they range in size from about 50-750 μm in diameter. Based on previous work by Gotoh2 and Okuchi3, a dual-echo white-blood (WB, 1st echo) and black-blood (BB, 2nd echo) imaging technique was developed. It is shown that using the complementary and inherently co-registered WB and BB images simultaneously is helpful in visualizing the LSAs and ascertaining their extent and tortuosity.
Methods
The vendor supplied multi-echo, three-dimensional (3D) spoiled gradient-recalled echo (SPGR) sequence was modified to acquire two echoes. The first echo is flow-compensated along all axes, but only at the center of k-space. This requires explicit gradient moment nulling along the readout direction, but no further gradients along the phase-encode or slice-encode directions. After the first echo, flow-sensitizing (aka diffusion) bipolar gradient pulses were added on all axes (Figure 1).
A healthy 50 yr old male subject who provided written and informed consent was scanned on a 3T system (Discovery 750; GE Healthcare, Waukesha, WI) using a 32-channel, receive-only head phased-array coil with the following parameters: 3D dual-echo flow-compensated SPGR, axial orientation, TR of 29.8 ms, TE1/TE2 of 7.2/23.4 ms, flip angle of 15º, field of view of 205x154 mm2, acquisition matrix of 320x240 (interpolated to 512x384), 80 slices at 0.8 mm thick (interpolated to 0.4 mm), b-value of 4 s/mm2, 0.75 averages, and parallel imaging with an acceleration factor of 1.25. The acquired voxel size was 0.64 x 0.64 x 0.8 mm3, the reconstructed voxel size was isotropic at 0.4 mm3, and the scan time was 5:50.
For visualization of the LSAs, the WB and BB 3D axial volumes were first reformatted in the coronal directions. Thereafter, maximum intensity projection (MIP) and minimum intensity projection (MinIP) of the WB and BB volumes, respectively, were done using 4-mm sections.
Results
Figure 2 shows an example of the WB MIP and BB MinIP pairs. The LSAs are faint and subtle on the WB MIP image, but are more clearly seen on the BB MinIP image. BB MinIP images are more sensitive to flow than the WB MIP images (due to the flow-sensitive diffusion pulses) but are non-specific – arteries and veins are equally nulled. Conversely, WB MIP images are more specific in depicting arteries (due to an in-flow effect) but their sensitivity is low. When used together, however, the LSAs are better visualized and characterized.
Albeit not shown here, the true benefit comes about when the user can interactively page between different slices to follow the LSAs and ascertain both the in-plane and through-plane extent and tortuosity.
Conclusions
It was demonstrated that a dual-echo white-blood and black-blood imaging technique at 3T marries the highly specific but lowly sensitive WB images with the lowly specific but highly sensitive BB images to help better visualize and delineate the lenticulostriate arteries.
Acknowledgements
This research was supported by the Canadian Institutes for Health Research and the Hopewell Professorship in Brain Imaging.
References
1. Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80(4):844-866.
2. G Gotoh K, Okada T, Miki Y, et al. Visualization of the lenticulostriate artery with flow-sensitive black-blood acquisition in comparison with time-of-flight MR angiography. J Magn Reson Imaging 2009; 29(1):65-69.
3. Okuchi S, Okada T, Ihara M, et al. Visualization of lenticulostriate arteries by flow-sensitive black-blood MR angiography on a 1.5T MRI system: A comparative study between subjects with and without stroke. AJNR Am J Neuroradiol 2013; 34(4):780-784.
Figures
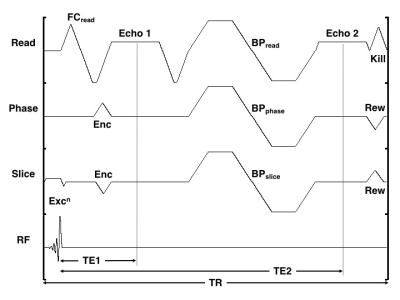
Figure 1: Timing diagram of the dual-echo WB and BB imaging technique. The readout (Read), phase-encode (Phase), slice-encode (Slice) and radio frequency (RF) axes are shown. FC denotes the explicit flow-compensating gradient along the readout direction, Excn denotes the slice-selective excitation, Enc and Rew denote the encoding and rewinding gradients, respectively, BP denotes the bipolar diffusion pulse, and Kill is the end-of-sequence killer. Echo1 occurs at time TE1, Echo2 occurs at time TE2, and the sequence repetition time is TR.
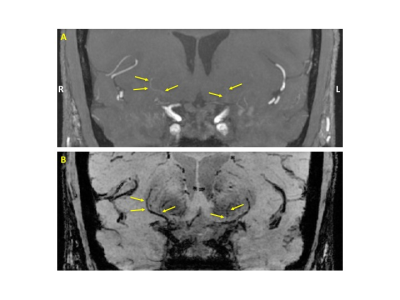
Figure 2: (A) MIP of the reformatted coronal WB image. (B) MinIP of the reformatted coronal BB image. R and L denote the anatomical right and left sides of the head, respectively. The LSAs are highlighted with arrows. The LSA on the right (3 arrows) is faint yet still clearly seen on the WB image, although its extent and conspicuity are better appreciated on the BB image. Conversely, the LSA on the left side (2 arrows) is barely visible on the WB MIP, but easily seen on the BB image.