1890
Quantitative Inhomogeneous Magnetization Transfer (ihMT) in Acute Stroke: A Preliminary Study1GE Healthcare, Taipei, Taiwan, 2GE Healthcare, Beijing, China, 3Department of Radiology, Shuang-Ho Hospital, Taipei Medical University, Taipei, Taiwan
Synopsis
Inhomogeneous magnetization transfer (ihMT) has been recent developed and has shown promise for myelin-specific imaging. The abnormal lipid pattern in the myelin of the white matter has been observed and could play an important role on ischemic lesion after stroke. The aim of this study was to investigate the myelin change within ischemic lesions using ihMT. In our presentative case, the abnormal area on DWI appears larger than that on ihMT. The difference may result from heterogeneous tissue characteristic in acute ischemic brain, which might evolve with the time after symptom onset and indicate a different clinical outcome.
Purpose:
The clinical significance of heterogeneity of brain damage within ischemic stroke lesion is unknown. The abnormal lipid pattern in the myelin of the white matter has been observed1 and could play an important role on ischemic lesion after stroke. A new advanced MRI technique named inhomogeneous magnetization transfer (ihMT) has been recent developed2,3. In contrast to conventional MT which is known to exhibit specificity limitation4, ihMT has been shown promise for myelin-specific imaging2. The aims of this study were first to established high quality and clinical feasible ihMT protocol and apply quantitative ihMT to investigate the myelin change within ischemic lesions.Methods:
All MRI acquisitions were performed on a 3T clinical scanner (Discovery MR750, GE Healthcare, Milwaukee, USA) using a 8-channel brain coil as the signal detection and whole body coil for radio-frequency excitation. The sequence of ihMT using a 3D spoiled gradient echo (SPGR), with a 5 ms Fermi pulse with peak B1 of 45 mG and ±5kHz offset prior to excitation. The scanning parameters are as follows: TR/TE=10.1/1.7 ms, field-of-view= 25.6 cm:, slice thickness=3.2 mm, matrix=128×96, scan time= 4 min 44 s. Quantification of ihMT used inverse subtraction methods and high tip angle reference scan. With an SPGR sequence in the short TR and low-tip regime, the difference in longitudinal relaxation rates observed with different MT pulse conditions was approximated by
Δ(R1*) =(cα2/2TR)ScΔ(1/S*)
where R1* is the longitudinal relaxation rate during particular MT; S* is the measured signal during this state. C is the flip angle scale factor for the high tip angle reference (C=4), α is the flip angle (α=8°), and Sc is the measured reference signal.
Results:
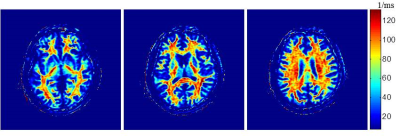
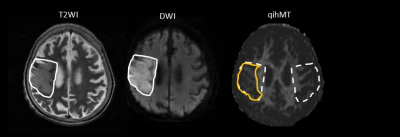
Quantitative ihMT images of a healthy volunteer with optimized protocol were shown in Fig. 1. Enhanced specificity of the quantitative ihMT signal for myelinated tissue is found as intense signal observed in highly myelinated white matter structures with little signal present in gray matter and negligible signal from the scalp. Representative T2-weighted images (T2WI), diffusion-weighted images (DWI), and ihMT of a 90-year-old stroke patient was obtained 60 hours after symptom onset (Fig. 2). After coregistration, the region-of-interest (ROI) of the restricted water diffusion on DWI was manual delineated and applied to T2WI and ihMT. The ROIs were mirrored to the contralateral normal-appearing hemisphere for unaffected controls. The area of restricted water diffusion appears larger than that of ihMT and T2WI.Discussion and Conclusion:
The imaging contrast provided by the ihMT uniquely delineate myelin-containing tissue and reflects the difference in saturation transfer between single- and dual-frequency off-resonance saturation. Ischemic stroke generally involves white matter. The clinical implication of the mismatch between areas of abnormal signal intensities on DWI and ihMT is unknown. In our presentative case, the abnormal area on DWI appears larger than that on ihMT. The difference may result from heterogeneous tissue characteristic in acute ischemic brain, which might evolve with the time after symptom onset and indicate a different clinical outcome.Acknowledgements
No acknowledgement found.References
1. Wender, M., et al., “Myelin lipids in ischemic stroke,” Neuropatol Pol, 29:87-94, 2014.
2. Varma, G., et al., “Magnetization Transfer from Inhomogeneously Broadened Lines: A Potential Marker for Myelin,” Magn Reson Med, 73:614–622, 2015.
3. Girard, OM., et al., “Magnetization Transfer from Inhomogeneously Broadened Lines (ihMT): Experimental Optimization of Saturation Parameters for Human Brain Imaging,” Magn Reson Med, 73:2111–2121, 2015.
4. Stroman, PW., et al., “The current state-of-the-art of spinal cord imaging: methods. Neuroimage, 84:1070-81, 2014.