1883
Microstructural Characterization of Post-Stroke Lesions in the Posterior Limb of the Internal Capsule in Subacute Patients using DTI and NODDIAlfonso Mastropietro1, Lucia Fontana2, Maria Luisa Malosio3,4, Laura Straffi5, Simona Marcheselli5, Marco Grimaldi2, and Giovanna Rizzo1
1Institute of Bioimaging and Molecular Physiology, Consiglio Nazionale delle Ricerche, Segrate, Italy, 2Neuroradiology Unit & Neuro Center, Humanitas Clinical and Research Center, Rozzano, Italy, 3Institute of Neuroscience, Consiglio Nazionale delle Ricerche, Milano, Italy, 4Laboratory of Brain Pathology and Pharmacology, Humanitas Clinical and Research Center, Rozzano, Italy, 5Stroke Unit & Neuro Center, Humanitas Clinical and Research Center, Rozzano, Italy
Synopsis
The purpose of his work was to characterize the Posterior Limb of the Internal Capsule (PLIC) of subacute stroke patients using both DTI and NODDI approaches to investigate microstructural changes occurring in the lesioned with respect to the unlesioned hemisphere. Six patients having a brain damage involving the Corticospinal Tract (CST) were enrolled. MRI was carried out on a 3T scanner about 14 days after stroke occurrence. DTI and NODDI analysis showed CST alterations in subacute stroke patients. FA and ODI were the only parameters that underwent significant modifications in PLIC regions.
Introduction
Diffusion MRI is a useful tool to evaluate alterations occurring in the whole brain in white matter after stroke. In fact, there is growing evidence that information about corticospinal tract (CST) integrity, and in particular at the level of the Posterior Limb of the Internal Capsule (PLIC)1, can improve predictions on patients’ motor outcome and the size of CST injury evaluated using DTI can be a prognostic factor that determines motor performance and outcome2. Recently, a more specific and advanced approach based on NODDI3 was applied in adult patients4 and in children5 during acute stroke. These preliminary results suggest that the quantitative parameters estimated by NODDI might be more specific markers of white matter re-arrangement after stroke and might provide valuable imaging biomarkers to assess brain microstructural changes with respect to classic DTI. Despite these preliminary applications, no studies, currently, have been focused on the evaluation of the brain in the subacute phase after stroke. The purpose of this work was to characterize the PLIC of subacute stroke patients, using both DTI and NODDI approaches, in order to investigate microstructural changes occurring in the lesioned with respect to the unaffected hemisphere.Methods
Methods Six stroke patients having a brain damage involving the CST were enrolled in this study (3M and 3F, age: 67±9). The PLICs of the patients were sites of stroke lesions. MRI sessions were carried out about 14±2 days after the stroke occurrence. Images were acquired on a Siemens MAGNETOM Verio 3T MRI scanner. The whole MRI protocol was composed by T1-weighted MPRAGE and 3D FLAIR sequences as anatomical reference and two different EPI sequences acquired with 2 shells (b=700 with 20 gradient directions / 3 b=0 and b=2000 s/mm2 with 64 gradient directions / 9 b=0) for DTI and NODDI analysis. The specific parameters for the EPI sequences were: TR=16900 ms, TE=96 ms, NA=1, matrix=128*128, number of slices=64, slice thickness=2 mm, in-plane resolution=2 mm2. Diffusion images were preprocessed and DTI maps (Fractional Anisotropy - FA and Mean Diffusivity - MD) were calculated using the software ExploreDTI. NODDI parameters (Orientation Dispersion Index - ODI, Intracellular Volume Fraction - Ficv, Extracellular Volume Fraction - Fecv and Isotropic Fraction - Fiso) were estimated using the dedicated Matlab toolbox. All the quantitative maps were coregistered to the MNI-152 space brain atlas using FSL before the ROIs definition. ROIs were manually drawn on the PLIC in both the ipsilateral and contralateral hemisphere with respect to the lesion. Statistical analysis was performed in Matlab using a Wilcoxon signed rank test. Tests with a p<0.05 were considered significant.Results
Figure 1 shows DTI and NODDI parameters maps estimated in one case of subacute stroke patient. FLAIR images clearly show a hyperintense signal corresponding to the site of the lesion involving the CST. Quantitative parameters are shown for PLIC in figure 2. As to the DTI parameters, FA significantly decreased in the PLIC of the ipsilateral hemisphere compared to the contralateral unlesioned side (from 0.67±0.03 to 0.46±0.06). Conversely, MD did not exhibit any significant alteration. As to NODDI parameters, Ficv, Fecv and Fiso did not show a significant modification in the ipsilateral hemisphere with respect to the contralateral one. However, ODI increased significantly in the ipsilateral PLIC (from 0.15±0.01 to 0.27±0.03).Discussion
The FA reduction in PLIC is in agreement with the decrease observed in the CST after acute stroke reported by the literature1-2. The observed concomitant increase of the ODI parameter of the NODDI suggests that the reduction of FA may be due to the increased dispersion of the white matter fibers in the affected hemisphere. Our work sustains and extends previous results present in the literature focused on the application of NODDI in the acute stroke phase. In those works, an increase of ODI as well as Ficv was shown in the lesioned areas in both adults and children. Our study does not show significant changes in Ficv in the ipsilateral PLIC with respect to the contralateral one. This could be explained by the subacute phase considered in the present work, which is characterized by a recovery of the axonal swelling that should reduce the impact of the intracellular compartment.Conclusion
DTI and NODDI analysis of diffusion MR images show CST alterations in subacute stroke patients. FA and ODI were the only parameters that underwent significant modifications in PLIC regions. We conclude that NODDI can provide a more detailed interpretation of the brain microstructural properties than the classical DTI approach.Acknowledgements
The work was partially supported by the project "EMpatia@Lecco" funded by Lombardy Region and Fondazione Cariplo, call Emblematiche Maggiori 2016, Provincia di Lecco.References
- Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prados F, Remollo S, Prats-Galino A, Soria G, Boada I, Castellanos M, Serena J. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011 May;32(5):857-63.
- Puig J, Blasco G, Schlaug G, Stinear CM, Daunis-I-Estadella P, Biarnes C, Figueras J, Serena J, Hernández-Pérez M, Alberich-Bayarri A, Castellanos M, Liebeskind DS, Demchuk AM, Menon BK, Thomalla G, Nael K, Wintermark M, Pedraza S. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology. 2017 Apr;59(4):343-351.
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain.Neuroimage. 2012 Jul 16;61(4):1000-16.
- Adluru G, Gur Y, Anderson JS, Richards LG, Adluru N, DiBella EV. Assessment of white matter microstructure in stroke patients using NODDI. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:742-5. 5.
- Joseph Y Yang, Richard Beare, Belinda Stojanovski, Wirginia J Maixner, Marc L Seal, Mark T Mackay. Characterizing Brain Microstructural Changes in Childhood Arterial Ischemic Stroke Using Multi-shell Diffusion Magnetic Resonance Imaging. Stroke. 2017;48:AWMP109, originally published February 21, 2017
Figures
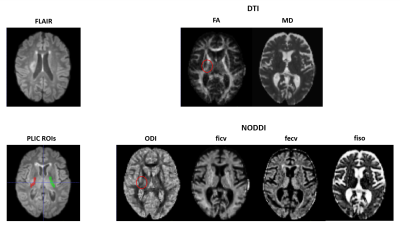
Figures on the left display the ischemic lesion on a FLAIR image (upper panel) and the ROIs drawn on the ipsilateral and contralateral PLIC (bottom panel). On the right, the parametric maps obtained by DTI (upper panels) and NODDI (bottom panels) are displayed. The red circle highlights the ischemic side.
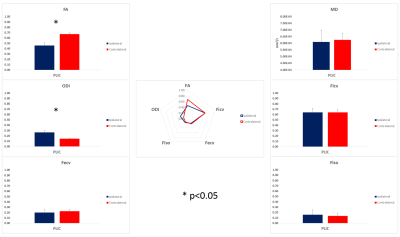
Quantitative comparisons of DTI and NODDI parameters are displayed. Asterisk is used to indicate significant differences (p<0.05).