1881
Simultaneous measures of brain oxygenation and perfusion using a 9.4T MRI in rats1Radiology, University of Calgary, Calgary, AB, Canada, 2Casualty Management Section, Defence Research and Development Canada- Suffield Research Centre, Suffield, AB, Canada
Synopsis
We developed a novel method to simultaneously measure tissue oxygenation and cerebral blood flow. This technique combines chronically implanted fiber-optic oxygen sensors and continuous arterial spin labeling MRI. An added benefit is that one can measure oxygen while the animals are awake and freely moving.
Purpose:
Tissue partial pressure of oxygen (pO2) is a sensitive biomarker for oxygen delivery and utilization. Measuring regional pO2 is an important parameter to determine the balance between cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2). In normal physiological conditions, the neurovascular coupling response dictates CBF delivers adequate oxygen and glucose in proportion to metabolic activity1. These mechanisms can become uncoupled during pathophysiological processes. The objective of this study was to develop a method to simultaneously measure brain pO2 and CBF in the MRI.Methods:
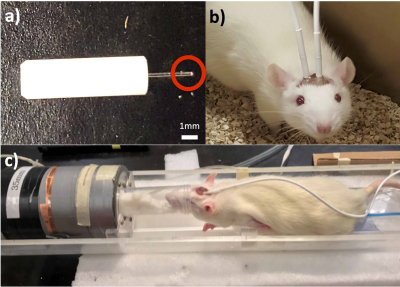
This research was conducted in accordance with the Canadian Council of Animal Care guidelines, under protocols approved by the University of Calgary Animal Care Committee. Male Sprague-Dawley rats (200-300g) were obtained from Charles-River. Fluorescent fiber-optic oxygen sensing probes (Fig. 1a) (Oxford Optronix, UK), were implanted in the motor cortex based on stereotaxic coordinates relative to the bregma. For the animals to fit in a 35 mm volume coil for better signal to noise ratio, the probes were chronically implanted at an angle in a location that ensures the probe tip is in the motor cortex. Following a week after surgery, pO2 measurements were taken in awake and freely moving rats for 3 consecutive days at 1 Hz for 10 minutes (Fig. 1b). MRI was performed on isoflurane-anaesthetized rodents in vivo on a 9.4T Bruker Avance console with a 35 mm volume coil (Fig. 1c). To account for magnetization transfer, a four image continuous arterial spin labeling (CASL) sequence was used: TR = 3000ms, TE = 2.66ms, averages: 16, RARE factor = 36, FOV = 30x30 mm, matrix = 128x128, voxel size: 0.23x0.23x1 mm.2 T1 map was obtained using a RARE-VTR sequence: TR=100, 500, 1000, 3000, 7500 ms, TE=10 ms. CASL sequence was used to quantify perfusion, while simultaneous pO2 measurements were taken for the duration of the scan (8 min).Results:
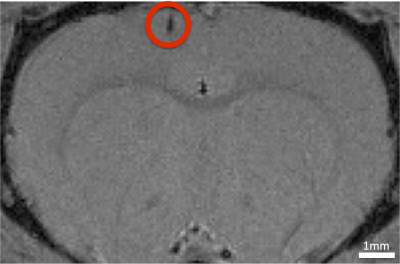
A gradient echo image was taken to show that the implant does not cause major susceptibility artifacts and the location of the probe (Fig. 2). The CBF measurements were not significantly different around the probe compared with the contralateral cortex (left motor cortex: 144±20 ml/100g/min and right motor cortex: 147±25 ml/100g/min) (Fig 3b). Fig. 3c shows, when comparing different animals, there is higher pO2 when CBF is higher.Discussion:
We were able to simultaneously measure pO2 using oxygen sensors and CBF using MRI, pO2 measures at high temporal resolution (2 Hz) but data can be averaged to match the timing of the perfusion MRI. A major advantage of chronically implanted probes is the ability to measure pO2 in awake and freely moving rats3 as well as within the MRI. Chronic implantation allows time for the tissue around the implant to recover3 which will give more confidence in studies of vascular regulation. Having a chronic implant ensures pO2 is measured in the same location and can be used for long-term studies.
The CBF near the probe was similar to that in the contralateral cortex indicating that the tissue had healed enough to reduce injury-related vascular dysregulation. When implanted acutely, using ketamine, much lower pO2 values were reported (6 mmHg)4. Acute implantations have also reported 25% lower values compared to recovery3. The pO2/CBF relationship would indicate that for a 50% increase in CBF, the pO2 would increase about 20-50%. This is within a range previously reported5. Although the power in this abstract is not sufficient to accurately describe the relationship, the example is sufficient to show that, in studies where abnormal neurovascular coupling is suspected, this type of study could provide novel data on the regulation of CBF.
There are several limitations associated with this technique. Trauma associated effects including inflammation and gliosis need to be considered. In a previous study, minimal inflammation and gliosis were detected3, indicating that the effects of trauma can be minimized by allowing the animals to recover. Another factor to consider is the effects of anaesthesia. Isoflurane has been shown to cause an increase in CBF and decrease in CMRO2, but the coupling response remains intact.6,7 Therefore, provided that baseline control measures are taken ahead of time, the changes in neuropathological conditions while under isoflurane may reflect changes when the animals are awake.
Conclusion:
A novel method was developed to simultaneously measure in vivo pO2 and perfusion. pO2 provides data on the balance between O2 delivery and utilization. Combining both gives an indication of coupling between O2 utilization and perfusion. A major strength is the ability to also measure pO2 while awake and freely moving, as well as within the MRI.Acknowledgements
This research was supported by the Department of National Defence. We thank our colleagues from the Defence Research and Development Canada- Suffield Research Centre for providing the materials necessary for the research.
We thank Brain Canada Platform grant for funding the research.
We thank Big Horn Sheet Metal, Canmore, AB, Canada for developing stereotaxic parts for the research.
References
1. Buxton, R. B., Wong, E. C. & Frank, L. R. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39, 855-864 (1998).
2. Lei, H. et al. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res 913, 174-179 (2001).
3. Ortiz-Prado, E., Natah, S., Srinivasan, S. & Dunn, J. F. A method for measuring brain partial pressure of oxygen in unanesthetized unrestrained subjects: the effect of acute and chronic hypoxia on brain tissue PO(2). J Neurosci Methods 193, 217-225, doi:10.1016/j.jneumeth.2010.08.019 (2010).
4. Sedlacik, J. et al. Correlation of oxygenation and perfusion sensitive MRI with invasive micro probe measurements in healthy mice brain. Z Med Phys 25, 77-85, doi:10.1016/j.zemedi.2014.01.004 (2015).
5. Demchenko, I. T., Mahon, R. T., Allen, B. W. & Piantadosi, C. A. Brain oxygenation and CNS oxygen toxicity after infusion of perfluorocarbon emulsion. J Appl Physiol (1985) 113, 224-231, doi:10.1152/japplphysiol.00308.2012 (2012).
6. Lenz, C., Rebel, A., van Ackern, K., Kuschinsky, W. & Waschke, K. F. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology 89, 1480-1488 (1998).
7. Hansen, T. D., Warner, D. S., Todd, M. M. & Vust, L. J. The role of cerebral metabolism in determining the local cerebral blood flow effects of volatile anesthetics: evidence for persistent flow-metabolism coupling. J Cereb Blood Flow Metab 9, 323-328, doi:10.1038/jcbfm.1989.50 (1989).
Figures