1876
Standard and Look-Locker FAIR-TrueFISP for arterial spin labelling on mouse at 9.4 T1Lund University Bioimaging Center, Lund University, Lund, Sweden
Synopsis
The study investigates TrueFISP readout for FAIR either as standard inversion recovery (IR) or as Look-Locker (LL) inversion recovery. These two methods are compared to EPI readout as implemented by Bruker. The aim was to show the improved image quality using TrueFISP and to evaluate the alternatives standard IR and LL. For FAIR-TrueFISP an in-house written method was created. The method was tested on a group of C57BL/6 mice at the field strength of 9.4 T. The results show cerebral blood flow maps with less distortion than EPI and the values found are in agreement with the literature.
Introduction
Pulsed arterial spin labelling sequences like FAIR generally suffer from weak signal to noise of the final perfusion signal. Thus the measurements often need long measurement times or lower resolution to compensate for this. A commonly used alternative for faster measurements is EPI-based FAIR, which is prone to geometrical distortions due to its sensitivity to inhomogeneous B0. With good shim this can, however, be compensated for and this also opens the way to an alternative readout technique for FAIR, i.e. TrueFISP1, which also suffers from inhomogeneous B0 at high field. In this study the FAIR TrueFISP is either implemented as classical inversion recovery (IR) or as Look-Locker inversion recovery (LL).Methods
Data were acquired on a 9.4 T Agilent magnet (Agilent, Santa Clara, USA) equipped with Bruker BioSpec AVIII electronics operating with ParaVison 6.0.1 and a BGA 12S HP gradient system (Bruker, Ettlingen, Germany). The coils used were a quadrature volume resonator (112/087) for transmission and a mouse brain phased array coil for reception. Both coils were from Bruker. All experiments were performed on C57BL/6 mice, which were either 6 weeks or 1 year old according to the ethical permit M132-13 approved by the Lund University Animal and Welfare Ethics Committee. The animals were anaesthetised with 1-2 % isoflurane using a 1:1 mixture of O2:N2O. The respiration was maintained at 82 ± 11 bpm and the temperature at 37.2 ± 0.4°C. Common parameters to the three protocols were a resolution of 233x234 μm2, a FOV of 17x15 mm2 and slice thickness of 1.5 mm. The inversion was achieved with an adiabatic pulse of 25 kHz bandwidth. Three different FAIR sequences were used: Bruker FAIR EPI (10.5 min, 4 averages) and an in-house written FAIR-TrueFISP, either as IR (16 min, 8 averages) or LL (10.5 min, 32 averages). The Bruker FAIR-EPI had TE of 11 ms, recovery time of 10 s and inversion times 0.03, 0.5, 1, 3, 5 and 10 s. The IR TrueFISP had TE of 0.9 ms, flip angle of 60°, centric encoding and sampling the same inversion times as above. The LL variant had flip angle of 7.5° and sampled 50 points over 9.54 s, the other parameters being the same as for IR. The perfusion maps were created in MATLAB2 according to Belle et al.3. For the LL the effective R1 was corrected according to Schmitt et al.4 assuming that flip angle and inversion degree were the same for selective and global inversion.Results and Discussion
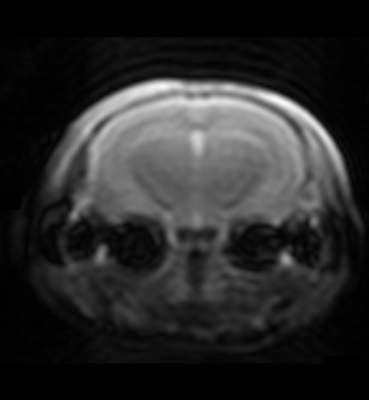
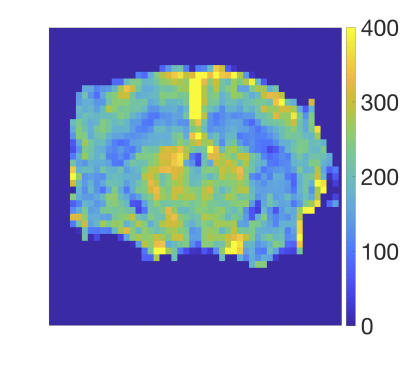
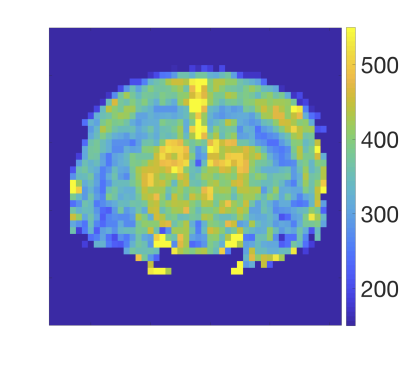
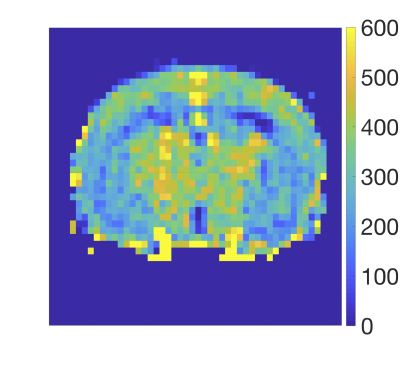
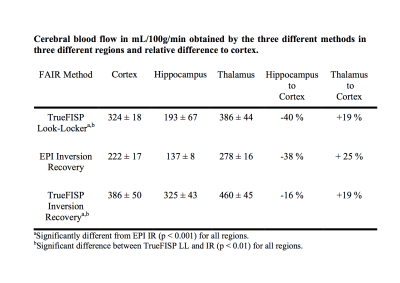
Figure 1 shows a typical image of the steady state magnetisation in a FAIR-TrueFISP of an adult mouse. As seen there are no banding artefacts in the brain. Figures 2-4 display cerebral blood flow (CBF) maps of 6-week-old mice (N=5) using FAIR-EPI, FAIR-TrueFISP IR and LL respectively. All three methods give clearly distinguishable perfusion, though of somewhat different amplitude. The TrueFISP images display no visible distortion in opposite to the EPI images. It is also seen in Figure 3, that in the LL sequence the hippocampal area located between the upper cortex area and the central thalamic area (both with higher perfusion amplitude), has more visible shine through of the ventricles. The actual position of the ventricles is also clearly visible in the individual R1-maps (not shown). A comparison of the CBF in the three different brain regions mentioned above is presented in Table 1. The relative change in perfusion in thalamus and hippocampus relative to cortex is similar in LL TrueFISP and EPI but different in hippocampus in IR TrueFISP. The CBF values are in agreement with other perfusion studies on mice5,6,7.Conclusions
The preliminary results show that the perfusion FAIR-TrueFISP is clearly performable at 9.4 T in adult mice without image artefacts and that it generally has better image quality than EPI. The perfusion values obtained with TrueFISP are significantly higher than those obtained with EPI, but the perfusion contrast in the images is very similar.Acknowledgements
René in'T Zandt (Lund, Sweden) is acknowledged for inspiring this project and for valuable discussion.References
1. Martirosian P, Klose U, Mader I, Schick F. FAIR True-FISP Perfusion Imaging of the Kidneys. MRM 2004; 51:353-361.
2. R2017a, The MathWorks Inc.
3. Belle V, Kahler E, Waller C, Rommel E, Voll S, Hiller KH, Bauer WR, Haase A. In Vivo Quantitative Mapping of Caridac Perfusion in Rats Using a Noninvasive MR Spin-Labeling Method. JMRI 1998; 8:1240-1245.
4. Schmitt P, Griswold MA, Jakob PM, Kotas M, Gulani V, Flentje M, Haase A. Erratum to “Inversion Recovery TrueFISP: Quantification of T1, T2 and Spin Density”. MRM 2004; 52:698.
5. Gao Y, Goodnough CL, Erokwu BO, Farr GW, Darrah R, Lu L, Dell KM, Yu X, Flask CA. Arterial spin labelling-fast imaging with steady-state free precession (ASL-FISP): a rapid and quantitative prefusion technique for high-field MRI. NMR Biomed. 2014; 27:996-1004.
6. Kober F, Duhamel G, Cozzone PJ. Experimental comparison of four FAIR arterial spin labelling techniques for quantification of mouse cerebral blood flow at 4.7 T. NMR Biomed. 2008; 21:781-792.
7. Muir ER, Shen Q, Duong TQ. Cerebral Blood Flow MRI in Mice Using the Cardiac-Spin-Labeling Technique. MRM 2008; 60:744-748.
Figures