1864
Rapid estimation of myelin for diagnostic imaging (REMyDI): A clinical and histopathological validation in multiple sclerosis1Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden, 2Department of Radiology, Division of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden, 3Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Harvard Medical School, Boston, MA, United States, 5Synthetic MR, Stockholm, Sweden, 6Center for Medical Image Science and Visualization, Linköping University, Linköping, Sweden, 7Department of Medical Psychology, Karolinska University Hospital, Stockholm, Sweden, 8Department of Neurology, Karolinska University Hospital, Stockholm, Sweden
Synopsis
Multiple sclerosis is a chronic inflammatory and neurodegenerative disease characterized by demyelination. To follow patients longitudinally and monitor treatment response, there is a need for robust and tissue-specific imaging biomarkers reflective of the heterogeneous disease course. Here, we aimed to validate REMyDI as an MRI-based measure of myelin ex vivo and in vivo. Histopathologically, REMyDI correlates well with all three of the studied myelin staining methods. In vivo, REMyDI revealed a strong sensitivity in differentiating white matter as compared to normal appearing white matter with associations to both cognitive (information processing speed) and physical disability (Expanded Disability Status Scale).
Purpose
Multiple sclerosis (MS) is the leading cause of non-traumatic neurological disability in young adults. The hallmarks of MS are inflammation and demyelination with neuro-axonal loss.1 MRI is essential for the diagnosis of MS but quantitative MRI measures are lacking in clinical practice.1,2 Since the disease is chronic, quantitative markers with high tissue-specificity would be valuable to follow disease progression and monitor therapy efficacy.1,2
Synthetic MRI (SyMRI) provides relaxometry, multiple contrasts and robust volumetrics time-efficiently.3,4 The simultaneous PD-, R1- and R2-mapping has recently been used to also allow myelin quantification, called Rapid Estimation of Myelin for Diagnostic Imaging (REMyDI).3,4,5
We aimed to study the specificity and clinical value of REMyDI in MS by histopathologically validating the model and prospectively evaluating its correlations to cognitive and physical disability in vivo.
Methods
Study population: 71 MS patients diagnosed according to the latest diagnostic criteria,6 and 21 age- and gender-matched controls. Demography is further detailed in Figure 1. Cognitive and physical disability were assessed with Symbol Digit Modalities Test (SDMT) and Expanded Disability Status Scale (EDSS), respectively.
In vivo imaging: All participants were scanned on the same Siemens Trio 3.0 T MRI scanner (Siemens Healthcare, Erlangen, Germany) with a 12-channel head coil using a saturation-recovery turbo spin echo sequence,7 30/34 axial slices, voxel size 0.9×0.9×3.5/4.0 mm3, gap 50%, flip angle 120°, repetition time 4260 ms, echo times 22 and 100 ms, 4 averages (150/580/2000/4130 ms effective inversion times), GRAPPA factor 2, acquisition time 6:50/7:47 min. SyMRI (v. 11.0 Beta 4, Synthetic MR, Linköping, Sweden) was used to fit quantitative PD, R1 and R2 maps. Examples are found in Figure 2.
Ex vivo imaging: A coronal hemispheric frontal-parietal section (6.4×1.0×11.4 cm) from a 56-year-old male donor with SPMS and 31 years’ disease duration was also scanned on a Trio scanner. The specimen was scanned at room temperature (19 °C) using the in vivo parameters and a 32-channel head coil. A higher resolution scan was additionally acquired: 15 slices, voxel size 0.39×0.39×2.0 mm3, gap 75%, 6 averages, acquisition time 32:47 minutes, illustrated in Figure 3. In order to compensate for fixation and temperature effects, R1 and R2 were rescaled by a factor of 3.3 and 1.9 respectively.8
Histology: The specimen was embedded and frozen at -20 ºC, from which 10 µm whole-section slices were collected using a cryomacrotome and mounted. Three myelin stains and one general stain were applied: Luxol Fast Blue, Bielschowsky’s silver stain, anti-Proteolipid-1 immunohistochemistry and Hematoxylin-Eosin, respectively.9 Sections were scanned and images white balanced, grayscaled and finally registered to one-another, illustrated in Figure 3. Regions of interest (ROI) were segmented and the average optical densities extracted; ten in the normal appearing white matter (NAWM) and six in white matter lesions.
MRI tissue segmentations: Lesions were automatically segmented using the lesion probability algorithm in Lesion Segmentation Toolbox (LST 2.0.15, Technische Universität München, Munich, Germany) for Statistical Parametric Mapping (SPM12, University College London, UK) and then manually corrected by trained raters (R.O. and M.P.) and finally reviewed by a resident in radiology (T.G.).
MRI-based myelin model: The PD, R1 and R2 maps were used in a four compartment tissue model, as previously described,5,10 where myelin is indirectly quantified through the magnetization transfer effect of the fast relaxing myelin-bound water to surrounding protons. This exchange effect causes shortening of observable relaxation rates and a decrease in the observable proton density since myelin-bound water itself is not observable. Further details can be found in previous studies.3,5,6,10
Results
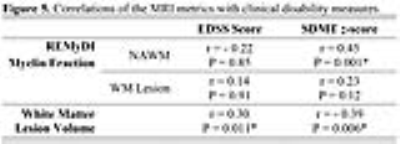
REMyDI myelin fraction correlated with all of the histopathological myelin staining methods, as shown in Figure 4. MS lesions had lower myelin fraction than the NAWM in the same subjects (81.90±3.06% 39.43±5.97%, P=1.1x10-65 by paired t-test). REMyDI myelin fraction in NAWM correlated with the information processing speed (SDMT). White matter lesion volume also correlated with EDSS and SDMT, values shown in Figure 5. Discussion/Conclusion
Myelin estimations based on REMyDI correlated well with histopathological myelin stainings. Furthermore, in vivo applications revealed high sensitivity to focal demyelination in MS lesions and also correlations with cognitive and physical disability. This suggests that REMyDI is a clinically applicable method for monitoring the dynamic demyelination and remyelination processes that take place throughout the disease course of multiple sclerosis and thus a suitable method for treatment studies aiming at enhancing remyelination.Discussion/Conclusion
Acknowledgements
We would like to thank the participants for making this study possible and the MRI staff at Karolinska University Hospital for their commitment. We would also like to thank Ani Varjabedian and André van der Kouwe for valuable advice on ex vivo procedures and imaging. The tissue specimen was provided by the Rocky Mountain MS Center Tissue Bank. The ex vivo histopathological examination and methodology was developed and administered by Invicro LLC under the supervision of Ildiko Polyak. This research was supported by the Stockholm City Council and Karolinska Institutet (ALF 20120213 and 20150166). Dr. Granberg was supported by the Swedish Society for Medical Research.References
1. Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, Inglese M, Guttmann CR, Horsfield MA, Filippi M. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7(7):615-625.
2. Filippi M, Rocca MA, Ciccarelli O, Stefano ND, Evangelou N, Kappos L, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15(3):292-303.
3. Blystad I, Warntjes JB, Smedby O, Landtblom AM, Lundberg P, Larsson EM. Synthetic MRI of the brain in a clinical setting. Acta Radiol. 2012;53(10):1158-1163.
4. Granberg T, Uppman M, Hashim F, Cananau C, Nordin LE, Shams S, Berglund J, Forslin Y, Aspelin P, Fredrikson S, Kristoffersen-Wiberg M. Clinical feasibility of synthetic MRI in multiple sclerosis: a diagnostic and volumetric validation study. AJNR Am J Neuroradiol. 2016;37(6):1023-9.
5. Warntjes M, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson in Med. 2008;60(2):320–9.
6. Polman, CH, Reingold, SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B. Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
7. Warntjes M, Persson A, Berge J, Zech W. Myelin detection using rapid quantitative MR imaging correlated to macroscopically registered luxol fast blue-stained brain specimens. AJNR Am J Neuroradiol. 2017;38(6):1096-1102.
8. Birkl C, Langkammer C, Golob-Schwarzl N, Leoni M, Haybaeck J, Goessler W, Fazekas F, Ropele S. Effects of formalin fixation and temperature on MR relaxation times in the human brain. NMR Biomed. 2016;29(4):458-65.
9. Kiernan JA. Histochemistry of staining methods for normal and degenerating myelin in the central and peripheral nervous systems. Journal of Histotechnology. 2007;30(2):87-106.
10. Warntjes M, Engström M, Tisell A, Lundberg P. Modeling the Presence of Myelin and Edema in the Brain Based on Multi-Parametric Quantitative MRI. Front Neurol. 2016;17(7):16.
Figures

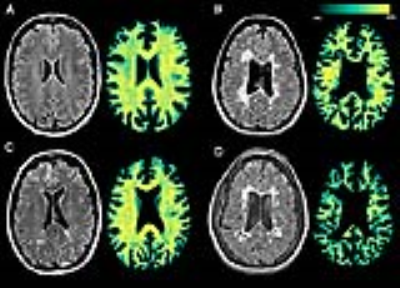
Synthetic T2-weighted FLAIR and the corresponding REMyDI myelin maps in four study participants:
A. 56-year-old female healthy control.
B. 39-year-old female RRMS patient, disease duration 14 years, EDSS score 1.0, SDMT z-score -1.00.
C. 53-year-old female PPMS patient, disease duration 25 years, EDSS score 7.0, SDMT z-score -0.38.
D. 40-year-old female SPMS patient, disease duration 9 years, EDSS score 3.5, SDMT z-score -3.89.