1862
mcDESPOT-derived measurements are sensitive to differences in myelin content and thickness in the corpus callosum of neuromyelitis optica patients and healthy controls1Neurology, University of British Columbia, Vancouver, BC, Canada, 2Radiology, University of British Columbia, Vancouver, BC, Canada, 3Neuroimaging, King's College London, London, United Kingdom
Synopsis
Histological studies suggest that white matter microstructure varies across different subregions of the corpus callosum (CC). We used mcDESPOT-derived measures to examine myelin content and thickness in vivo in 3 subregions of the CC in healthy controls (HC) and individuals with neuromyelitis optica spectrum disorder (NMOSD). Differences in both myelin content and thickness were observed in different subregions of the CC in HC. Myelin content was decreased in posterior CC in NMOSD relative to HC. mcDESPOT-derived myelin measurements are sensitive to differences in white matter microstructure and can be used to investigate the underlying pathology contributing to demyelinating diseases.
Introduction
The corpus callosum (CC) is the largest white matter fiber bundle in the brain and is the major commissural pathway connecting the two cerebral hemispheres.1 There is evidence demonstrating that white matter microstructure varies across different regions of the CC. Imaging studies have reported that myelin content is highest in the splenium relative to the genu and body,2 while histological studies show that myelin thickness is greater in the body relative to the genu and splenium of the CC.3
Multicomponent relaxometry is a noninvasive imaging method that can provide tissue-specific measurements in vivo, enabling investigation of myelin microstructure in healthy individuals as well as demyelinating diseases. Multicomponent Driven Equilibrium Single Pulse Observation of T1 and T2 (mcDESPOT)4 can be used to investigate myelin content and thickness in tissue. Specifically, the myelin water fraction (MWF) quantifies the amount of signal from water between the myelin bilayers, and is thus related to myelin content. Myelin water residence time (τM), which estimates the time taken for myelin water to exchange with intra/extracellular water, is expected to reflect myelin thickness.
In this study, we first sought to determine if MWF and τM would detect variations along the CC in healthy controls (HC) demonstrated by previous studies. In particular, we aimed to determine if τM would mirror variations in myelin thickness in the CC, in vivo, as seen in histological studies. We then applied these techniques to investigate normal appearing white matter (NAWM) in the CC in neuromyelitis optica spectrum disorder (NMOSD), which is a neurological disease similar in clinical presentation to multiple sclerosis (MS). We previously demonstrated that unlike MS, MWF is unaffected in NAWM for NMOSD.5 We hypothesized that we may be able to highlight more subtle pathological changes in NMOSD by segmenting the CC into 3 subregions and by examining τM in addition to MWF.
Goals: 1) To determine if quantitative, myelin-specific MRI metrics are sensitive to differences in white-matter microstructure across subregions of the CC in HC. 2) Using NMOSD as a test case, investigate whether myelin-specific MRI metrics can measure abnormalities in demyelinating diseases that have not previously been detected.
Methods
13 patients with NMOSD (9 female, mean age: 43y (range: 24-59y), median EDSS=2.5 (range: 1-6), mean disease duration=7.12y (range: 0.5-14y)) and 13 HC (9 female, mean age: 40y (range: 26-56y)) were included. Participants were scanned on a 3.0T Phillips Achieva system (Best, Netherlands) using a mcDESPOT protocol4 with 1.7mm3 isotropic voxel size. Voxel-wise mcDESPOT analysis yielded measurements of MWF and τM derived from a three-pool model.4 CC segmentation was performed using an atlas based approach to obtain regions of interest (ROI) for the normal-appearing genu, body and splenium. Mean MWF and τM were extracted from each ROI. One-way repeated measures analysis of variance (ANOVA) investigated within-group differences. The Mann-Whitney U test explored between-group differences.Results
Within HC, differences in both MWF and τM were observed between ROIs of the CC. MWF: genu 0.195 (0.017), body 0.196 (0.014), splenium 0.215 (0.009); p=0.005 (Figure 1). τM: genu 66.2 (9.3) ms, body 82.9 (10.9) ms, splenium 76.1 (11.0) ms; p=0.006 (Figure 2). In NMOSD, MWF in the body (p=0.04) and splenium (p=0.003) were significantly reduced compared to HC (Figure 3). No differences in τM were found between HC and NMOSD (Figure 4).Discussion
MWF and τM are in agreement with expected variations in HC CC myelin, supporting the interpretation of the measurements and establishing methodological sensitivity. MWF was significantly reduced in NMOSD compared to HC in the body and splenium with a decreased trend in the genu. τM was not significantly reduced in NMOSD compared to HC, implying preserved myelin thickness.Conclusion
mcDESPOT-derived myelin measurements are sensitive to differences in white matter microstructure and can be used to investigate the underlying pathology contributing to demyelinating diseases.Acknowledgements
We thank all study participants and the UBC MRI technologists for their contributions to this project, and are grateful to Susan Diamond, Rick Diamond, and the Milan & Maureen Ilich Foundation for their support.References
1. Fitsiori, A., Nguyen, D., Karentzos, A., Delavelle, J. & Vargas, M. I. The corpus callosum: white matter or terra incognita. Br J Radiol 84, 5–18 (2011).
2. Laule, C. et al. Magnetic resonance imaging of myelin. Neurotherapeutics 4, 460–484 (2007).
3. Aboitiz, F., Scheibel, A. B., Fisher, R. S. & Zaidel, E. Fiber composition of the human corpus callosum. Brain Res. 598, 143–153 (1992).
4. Deoni, S. C. L., Matthews, L. & Kolind, S. H. One component? Two components? Three? The effect of including a nonexchanging ‘free’ water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn Reson Med 70, 147–154 (2013).
5. Matthews, L. et al. Imaging Surrogates of Disease Activity in Neuromyelitis Optica Allow Distinction from Multiple Sclerosis. PLoS ONE 10, e0137715 (2015).
Figures
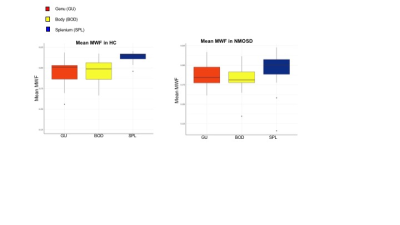
Figure 1: Mean MWF in HC and NMOSD
Significant differences in MWF were observed between ROIs of the CC in HC: genu 0.195 (0.017), body 0.196 (0.014), splenium 0.215 (0.009); p=0.005. No significant differences in MWF were observed between ROIs of the CC in NMOSD (p=0.3).
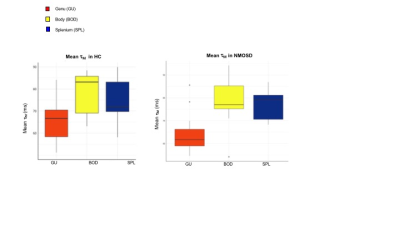
Figure 2: Mean τM in HC and NMOSD
Significant differences in τM were observed between ROIs of the CC in HC: genu 66.2 (9.3) ms, body 82.9 (10.9) ms, splenium 76.1 (11.0) ms; p=0.006.
Significant differences in τM were observed between ROIs of the CC in NMOSD: genu 64.3 (9.0) ms, body 79.1 (10.3) ms, splenium 77.0 (6.4) ms; p=0.001.
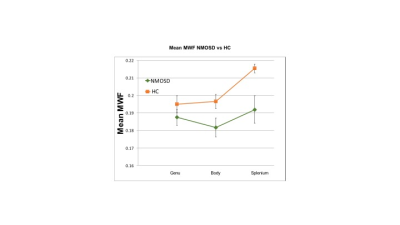
Figure 3: Mean MWF NMOSD vs HC
MWF in the body (p=0.04) and splenium (p=0.003) were significantly reduced in NMOSD compared to HC.
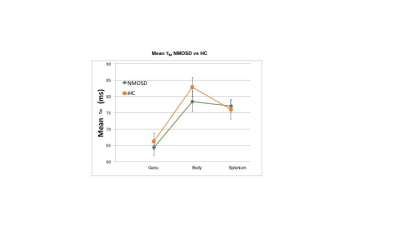
Figure 4: Mean τM NMOSD vs HC
No significant between group differences were found in τM.