1855
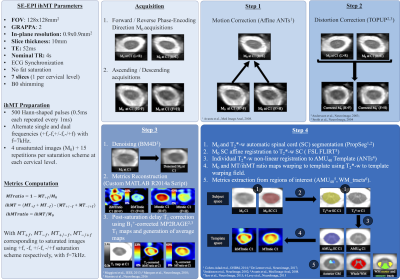
A new rapid and high-resolution multi-slice inhomogeneous Magnetization Transfer protocol to evaluate diffuse and regional cervical cord myelination at 3T1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3iLab-Spine International Associated Laboratory, Montreal, Marseille, France, 4Department of Neurology, CHU Timone, AP-HM, Marseille, France
Synopsis
The inhomogeneous Magnetization Transfer (ihMT) technique has recently been proposed as a new method to probe the cervical spinal cord (CSC) myelin-content. Studies reported so far were limited to single-slice acquisition, hence precluding investigation of the whole CSC within a short acquisition time. To overcome this limitation, a 2D multi-slice single-shot Spin-Echo-Echo-Planar Imaging (SE-EPI) read-out approach was implemented at 3T along with strategies to correct for inherent susceptibility-induced image-distortions and post-saturation relaxation effect for each slice. Validated on phantom and applied to healthy subjects and a patient with multiple sclerosis, this preliminary study shows the promising value of SE-EPI ihMT in the clinical context.
Introduction
Neuroimaging is a key factor in the accurate diagnosis, prognosis and disease monitoring of various SC pathologies, especially the demyelinating Multiple Sclerosis (MS)(1). Research efforts have particularly led to a large panel of advanced techniques allowing to perform in vivo myelin MR imaging (MWF, MT/qMT, g-ratio)(2–6). In-line with these developments, the inhomogeneous Magnetization Transfer (ihMT) technique has recently been proposed as a presumably more-specific myelin-marker (7–9), with preliminary results in both the brain (10, 11) and the cervical spinal cord (CSC) (9, 12–14) demonstrating a good myelin-sensitivity. However, cross-sectional ihMT studies reported so far in the CSC were limited to single-slice acquisitions based on HASTE (half-Fourier acquired single-shot turbo spin-echo) read-out, hence preventing investigation of the whole CSC within a short acquisition time. In this study, we present a multi-slice 2D single-shot Spin-Echo Echo-Planar Imaging (SE-EPI) ihMT protocol, devoid of susceptibility-induced image-distortions and T1 relaxation effects, covering from C1 to C7 levels, compatible with a clinical scan-time (10 minutes). Combined with dedicated SC templates (AMU40(15), WM_tracts(16)), this protocol allowed for sub-millimetric regional and diffuse MT/ihMTratios quantification across the CSC.Methods
Experiments were performed on a 3T Siemens Verio scanner using standard coils. Main parameters for the SE-EPI ihMT sequence are reported in Fig1. In addition to forward/reverse phase-encoding direction acquisition of unsaturated (M0) datasets for EPI-induced distortion correction, ascending/descending ihMT-prepared slice read-outs were acquired to mitigate the post-saturation relaxation effect occurring in each slice. B1+-field and MP2RAGE T1-mapping acquisitions (17, 18) were additionally performed for further T1 relaxation effect correction. Total scanning time for the 3 sequences was about 18 minutes. A 2D multi-gradient-echo sequence was also acquired for WM/GM segmentation. Data post-processing pipeline including denoising, distortion and motion correction, relaxation compensation and co-registration within a normalized space is also presented in Fig1. Validation of the technique was first performed on a lipid-based phantom (hair-conditioner). Then, 2 groups of 5 healthy subjects (G1: age 28.8±4.3yo; G2: age 60.2±2.9), as well as a relapsing-remitting MS patient (22yo, EDSS:1, disease duration: 2 years) were scanned. Two healthy subjects were scanned at two additional time points to evaluate reproducibility.Results
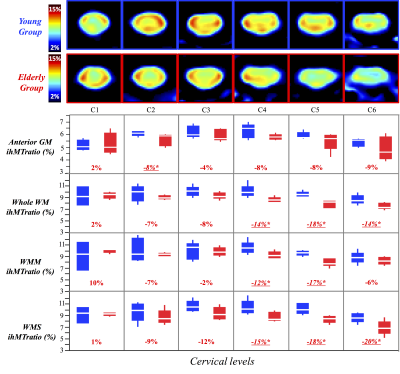
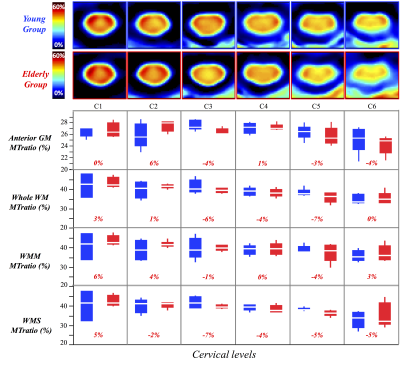
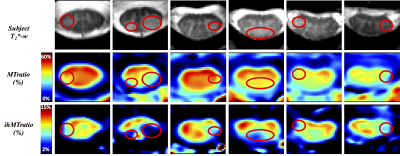
In silico and in vivo experiments led to reproducibility in the ihMT/MT measurements of 97%/99% and 87%/93% respectively. Fig2 and Fig3 show average ihMTratio and MTratio maps, respectively, from C1 to C6 for the two age-groups (the C7 level was not included in the present study due to non-systematic image quality (B1+ drop out, altered shim or fat contamination)). Preliminary inter-group comparisons in the different regions of interest (anterior gray matter (GM), whole white matter (WM), motor and sensory tracts) are also reported. These preliminary results showed a significant decrease of ihMTratio with age in lower levels, in both motor and sensory tracts, in line with previous study using HASTE read-out (13), whilst no significant change of MTratio was found. Fig4 illustrates MT/ihMT C1-to-C6 maps from the MS patient showing important decrease of ihMTratio in both normal-appearing and lesioned regions, as compared to young healthy subjects (average decrease of 27%), whilst moderate changes were found in MTratios (15% decrease in lesions as compared to healthy subjects values), suggesting a higher or earlier myelin-sensitivity of the ihMTratio as compared to MTratio.Discussion / Conclusion
Accurate quantification of ihMT within the spinal cord currently requires ECG-synchronization (9), which is not yet fully compatible with 3D imaging. As an alternative to cover the whole cervical spinal cord, a multi-slice 2D imaging/processing strategy has thus been developed, allowing to collect reliable ihMT data from C1 to C6, within a scan-time compatible with clinical studies. Current methodology is under improvement to correct for B1+ inhomogeneity (16% drop-out in average at C7 level). Since fat saturation or outer volume suppression are currently not compatible with the ihMT preparation, a reduced FOV acquisition would also help minimizing fat artefact and aliasing from the neck. Despite these technical challenges, these preliminary applications on both healthy and pathological subjects already showed promising results in myelination/demyelination quantification in spinal cord tissue. This technique, which should of course be compared with other myelin imaging techniques, could be of great interest as a myelin biomarker especially in longitudinal studies and for early prognosis.Acknowledgements
This study was supported by the French CNRS (Centre National de la Recherche Scientifique) and the French MESR (Ministère de l'Enseignement Supérieur et de la Recherche).
The authors are grateful for the time and efforts invested in this study by the patients, healthy volunteers, caregivers and clinicians of La Timone Hospital. They would also like to thank Guilherme Ribeiro for MATLAB code optimization, Veronique Gimenez, Lauriane Pini, Elisabeth Soulier and Sylviane Confort- Gouny for study logistics, volunteer recruitment and help with data acquisition.
References
1. Caputi N, Passafiume D, Boller F: History of neuroimaging in Multiple Sclerosis: 1838-2016 (P2.038). Neurology 2017; 88(16 Supplement).
2. Battiston M, Grussu F, Ianus A, et al.: An optimized framework for quantitative magnetization transfer imaging of the cervical spinal cord in vivo. Magn Reson Med 2017.
3. Ljungberg E, Vavasour I, Tam R, et al.: Rapid myelin water imaging in human cervical spinal cord. Magn Reson Med 2017; 78:1482–1487.
4. Henkelman RM, Huang X, Xiang Q-S, Stanisz GJ, Swanson SD, Bronskill MJ: Quantitative interpretation of magnetization transfer. Magn Reson Med 1993; 29:759–766.
5. Stikov N, Campbell JS, Stroh T, et al.: In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 2015; 118:397–405.
6. Duval T, Levy S, Stikov N, et al.: g-Ratio weighted imaging of the human spinal cord in vivo. Neuroimage 2016.
7. Varma G, Duhamel G, de Bazelaire C, Alsop DC: Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med 2015; 73:614–22.
8. Varma G, Girard OM, Prevost VH, Grant AK, Duhamel G, Alsop DC: Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J Magn Reson 2015; 260:67–76.
9. Girard OM, Callot V, Prevost VH, et al.: Magnetization transfer from inhomogeneously broadened lines (ihMT): Improved imaging strategy for spinal cord applications. Magn Reson Med 2016.
10. Alsop DC, Dandamudi R, Bakshi R: Inhomogeneous Magnetization Transfer Imaging of Myelin Concentration in Multiple Sclerosis. Proceedings of ISMRM, 2007.
11. Van Obberghen E, Le Troter A, Prevost V, et al.: Inhomogeneous Magnetization Transfer (ihMT) in normal-appearing tissue correlates with disability of multiple sclerosis patients. (P4.360). Neurology 2017; 88(16 Supplement).
12. Rangwala N, Varma G, Hackney D, Alsop DC: Quantification of myelin in the cervical spinal cord using inhomogeneous magnetization transfer imaging. Proceedings of ISMRM, 2013.
13. Taso M, Girard OM, Duhamel G, et al.: Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed 2016; 29:817–32.
14. Rasoanandrianina H, Grapperon A-M, Taso M, et al.: Region-specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed :e3801–n/a.
15. Taso M, Le Troter A, Sdika M, et al.: A reliable spatially normalized template of the human spinal cord - Applications to automated white matter/gray matter segmentation and tensor-based morphometry (TBM) mapping of gray matter alterations occurring with age. Neuroimage 2015; 117:20–8.
16. Levy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J: White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage 2015; 119:262–271.
17. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R: MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010; 49:1271–81.
18. Massire A, Taso M, Besson P, Guye M, Ranjeva JP, Callot V: High-resolution multi-parametric quantitative magnetic resonance imaging of the human cervical spinal cord at 7T. Neuroimage 2016; 143:58–69.
Figures