1853
Sequential Changes of Diffusion Anisotropy and Mean Kurtosis in Cuprizone-Induced Demyelination: A Rat Model1Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Medical Imaging, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan, 3Research Center of Translational Imaging, College of Medicine, Taipei Medical University, Taipei, Taiwan, 4School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan, 5Department of Anatomy and Cell Biology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 6Graduate Institute of Biomedical Electrics and Bioinformatics, National Taiwan University, Taipei, Taiwan
Synopsis
The verification of cuprizone-induced demyelination in a rat model remains controversial. This study aims to develop a reliable cuprizone-induced demyelination rat model and to test the ability of DKI to monitor the sequential changes during brain demyelination. Our findings demonstrated that DKI could provide complementary information, associated with pathophysiological processes after demyelination in rat brain, which may have potential to detect microstructural changes at both acute and chronic stages and contribute to evaluations of further therapeutic strategies.
Introduction
Multiple sclerosis is a neurological disease, resulting from immune-mediated demyelination, that can affect brain, spinal cord and the optic nerves [1]. Cuprizone, a copper chelator, could lead to abnormal cell metabolism, demyelination, and oligodendrocytic and neuronal death ultimately. Although the cuprizone mouse model has been well-established for studying demyelinating processes, the interpretation of MR findings could be limited because of severe partial volume effect in the acquired images due to tiny size of the mouse brain [2]. On the other hand, the verification of cuprizone-induced demyelination in a rat model remains controversial, suggestive of urgent demand in developing this model for understanding the associated pathophysiological mechanism, particularly the temporal evolution, in rat. Additionally, diffusion kurtosis imaging (DKI) provides an alternative to assess the microstructural changes in rat brain with less sensitivity to the partial volume effect during the progression of demyelination compared with diffusion tensor imaging [3]. Therefore, the purpose of this study is to establish a reliable cuprizone-induced demyelination rat model and to test the ability of DKI to monitor the sequential changes during brain demyelination.Methods
Five Sprague Dawley (SD) rats (8 weeks of age) had stand rodent lab chow mixed with 0.6% w/w cuprizone (Sigma-Aldrich, USA) for 4 weeks to induce demyelination. The MR imaging was performed on a 7.0T animal MR scanner (PharmaScan, Bruker, Erlangen, Germany) with a 72 mm transmitting coil and a quadrature surface coil for receiving at 0 week (baseline), 2 weeks (acute stage) and 4 weeks (chronic stage) after the administration of cuprizone. After conventional T2-weighted imaging, DKI data was obtained using spin echo diffusion-weighted 2D segmented EPI with b values of 0, 1000, and 2500 s/mm2 in 30 non-collinear directions. Other imaging parameters were as follows: TR/TE = 2000/26.41 ms, FOV = 25x25 mm2, matrix size = 96x96 (zero-filled to 192x192), slice thickness = 1 mm, # of slice = 17, # of segment = 4, NEX = 2, and total scan time less than 1.5 hour. Fractional anisotropy (FA) and mean kurtosis (MK) maps were derived for further comparisons based on the previous algorithm. Moreover, immunohistochemical analysis was performed to assess the pathophysiological changes after brain demyelination.Results
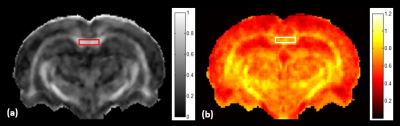
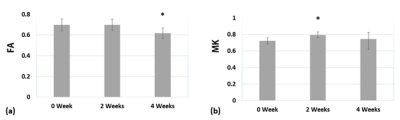
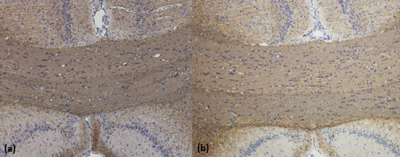
Results of the derived FA and MK maps from one rat before administration of cuprizone were shown in Figure 1. Since corpus callosum is the primary white matter in rat brain, one region-of-interest (ROI) was placed on the FA map covering the splenium of corpus callosum for quantitative analysis. The mean and standard deviations of the derived parametric indices at 0 week, 2 weeks, and 4 weeks after administration of cuprizone were displayed in Figure 2. The MK value significantly elevated at the acute stage and the FA value significantly decreased at the chronic stage after cuprizone-induced demyelination compared with them at the baseline (P < 0.05). Figure 3 shows the immunohistochemical analysis of the myelin basic protein (MBP) in rat brains. Dark brown color on the corpus callosum reveals a more dense MPB content in a control rat than that in a cuprizone-induced SD rat (b).Discussion
To our best knowledge, this is the first report to successfully develop cuprizone-induced demyelination in SD rats with histological confirmation. This study demonstrated the feasibility of DKI method for monitoring the progression of cuprizone-induced brain demyelination in a rat model. Recently, several reports have indicated that DKI could provide more comprehensive information about brain microstructures and heterogeneity compared to conventional DTI method. In our preliminary result, a significantly decrease of FA in the corpus callosum was observed at 4 weeks after administration of cuprizone, suggesting the occurrence of demyelination. Additionally, a significant elevation of MK could result from the reactive astrogliosis due to the inflammatory events at the acute stage [4]. To sum up, DKI could provide complementary information, associated with pathophysiological processes after demyelination, which may have potential to detect microstructural changes in both acute and chronic stages and contribute to evaluations of further therapeutic strategies.Acknowledgements
This study was supported by the Ministry of Science and Technology, Taipei, Taiwan (MOST 106-2221-E-038-002).References
1. Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502-17.
2. Falangola MF, Guilfoyle DN, Tabesh A, et al. Histological correlation of diffusional kurtosis and white matter modeling metrics in cuprizone-induced corpus callosum demyelination. NMR Biomed 2014;27(8):948-57.
3. Hui ES, Cheung MM, Qi L, Wu EX. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. Neuroimage 2008;42(1):122-34.
4. Zhuo J, Xu S, Proctor JL, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 2012;59(1):467-77.
Figures