1849
Three-Dimensional Inversion Recovery Ultrashort Echo Time (3D IR-UTE) Magnetic Resonance Imaging of Myelin in Rats and Mice Subject to Cuprizone Treatment1University of California, San Diego, San Diego, CA, United States, 2VA San Diego Healthcare System, San Diego, CA, United States, 3Genomics Institute of the Novartis Research Foundation (GNF), San Diego, CA, United States
Synopsis
Ultrashort echo time (UTE) MRI is capable of directly imaging myelin protons. We present the first application of a UTE sequence to study an animal model of demyelination, using inversion recovery (IR) and 3D radial sampling. Mice treated with 0.2% cuprizone for 5 weeks show loss of the 3D IR-UTE signal in the lateral corpus callosum, which is expected to be maximally demyelinated at this time point. Future studies of histologically validated demyelination and remyelination in this model will further confirm the capability of 3D IR-UTE to selectively image myelin.
Introduction
Myelin is a multi-lamellar membranous structure consisting of alternating protein and lipid layers 1. Direct imaging of myelin may be important for the diagnosis and assessment of many neurological diseases such as multiple sclerosis (MS). However, myelin protons have extremely short T2s on the order of hundreds of microseconds or less, and cannot be directly imaged with conventional magnetic resonance imaging (MRI) sequences 2-5. Inversion recovery prepared ultrashort echo time (IR-UTE) sequences with TEs in the order of tens of microseconds can potentially directly image myelin protons, with long T2 water signals being suppressed through adiabatic inversion and signal nulling 3-6. This study aimed to develop a 3D adiabatic IR-UTE imaging sequence for selective imaging of myelin in white matter of small animal brains and to implement this sequence at 7T on the cuprizone mouse model of MS (toxin-induced demyelination and remyelination).Methods
Two normal rats and ten C57BL/6 mice (five treated with 0.2% cuprizone chow for 5 weeks and five controls) were sacrificed for this study. This cuprizone dose and duration has been well-established to induce maximal regional demyelination in the corpus callosum in this mouse strain 7.
Brain imaging was performed on a Bruker 7T scanner. A rat/mouse brain coil was used for signal reception. A conventional T2-weighted fast spin echo (T2-FSE) sequence, with TR = 2760 ms, TE = 40 ms, and echo train length = 8, was used to image the long T2 signals including gray and white matter in the brain. A conventional two-dimensional adiabatic inversion recovery prepared FSE (2D IR-FSE) sequence was used to measure T1 of long T2 white matter. A 3D Cones IR-UTE sequence was used to image myelin, using the following parameters: TR = 1000 ms, inversion time (TI) = 350 ms, TE = 0.2 ms, FOV = 2.2×2.2×2.2 cm3, matrix = 128×128×128 cm3, flip angle (FA) = 20º. The acquired voxel size = 172×172×172 µm3. To speed up data acquisition, a total number of spokes of 21 were acquired per IR preparation, leading to a total scan time of 100 min. The same 3D IR-UTE sequence was repeated with two different TEs of 0.02 ms and 2.0 ms. Similar imaging parameters were used for the T2-FSE and IR-FSE sequences.
Results
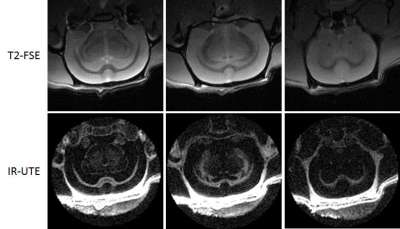
Figure 1 shows representative 2D T2-FSE and 3D IR-UTE images. White matter was depicted with low signal with the 2D T2-FSE sequence, which can only detect signal from long T2 components. The 3D IR-UTE sequence shows excellent contrast for myelin protons with efficient suppression of long T2 signals from white matter and gray matter. The skull was also depicted as high signal with the 3D IR-UTE sequence but near zero signal with the 2D T2-FSE sequence.
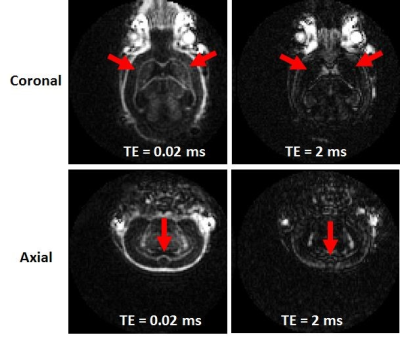
Figure 2 shows representative coronal and axial slices from 3D IR-UTE imaging of a normal mouse brain with two different TEs of 0.02 ms and 2 ms. Signal from white matter dropped to near noise level at a short TE of 2 ms, consistent with short T2 myelin protons being selectively detected by the IR-UTE sequence.
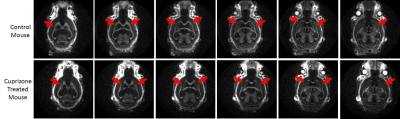
Figure 3 shows selected coronal slices from 3D IR-UTE imaging of a normal mouse and a mouse treated with 0.2% cuprizone for 5 weeks. The cuprizone treated mouse shows reduced myelin signal with regions of near zero signal, suggesting near complete myelin loss.
Discussion
This ex vivo rat and mouse study demonstrates that the 3D IR-UTE sequence provides selective volumetric imaging of myelin protons in white matter of the brain with uniform suppression of long T2 signals. The cuprizone treated mice show reduced myelin signal and regional loss of myelin in the lateral corpus callosum, consistent with previous studies 7. Correlation of the 3D IR-UTE signal with histologically confirmed demyelination and remyelination will provide more solid evidence about the capability of this sequence for direct imaging of myelin protons, and will be performed in follow-up studies.Conclusion
The 3D IR-UTE sequence holds potential for direct imaging of myelin protons, allowing further validation studies using the cuprizone rodent model with histological correlation. Direct imaging of myelin may significantly increase the sensitivity and, importantly, the specificity in detecting early changes in neurological diseases associated with demyelination and remyelination.Acknowledgements
The authors acknowledge grant support from the NIH (1R01 NS092650 and T32 EB005970), VA Clinical Science Research and Development Service (Merit Award I01CX001388), and GE Healthcare.References
1. Laule C, Vavasour IM, Kolind SH, Li DK, Traboulsee TL, Moore GR, MacKay AL. Magnetic resonance imaging of myelin. Neurotherapeutics 2007;4(3):460-484.
2. Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med 2011;66(1):24-31.
3. Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci U S A 2012;109(24):9605-9610.
4. Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, Corey-Bloom J, Bydder GM. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage 2014;87:32-41.
5. Sheth V, Shao H, Chen J, Vandenberg S, Corey-Bloom J, Bydder GM, Du J. Magnetic resonance imaging of myelin using ultrashort echo time (UTE) pulse sequences: phantom, specimen, volunteer and multiple sclerosis patient studies. Neuroimage 2016; 136: 37-44.
6. Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med 2016; 76(2):577-582.
7. Matsushima GK, Morrell P. The Neurotoxicant, Cuprizone, as a Model to Study Demyelination and Remyelination in the Central Nervous System. Brain Pathol. 2001; 11(1):107-116.
Figures