1848
Relevance of microglia receptor TREM2 for remyelination as revealed by multimodal MRI in the cuprizone mouse model1NORD Discovery & Translational Area, Pharmaceutical research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland
Synopsis
Demyelination and ensuing axonal damage are hallmarks of numerous neurodegenerative disorders. Novel treatment strategies seek to enhance remyelination and axonal recovery through acceleration of myelin debris clearance by phagocytic microglia. TREM2 is a receptor expressed by microglia that has been implicated in the regulation of phagocytosis, migration and anti-inflammatory activity. Here, we further elucidated the role of TREM2 in de- and remyelination processes by means of multiparametric in vivo MRI. We combined a TREM2 loss-of-function mouse model with cuprizone feeding as an accepted model for demyelination. Deficiency of TREM2 leads to progressive structural disintegration and absence of proper remyelination.
INTRODUCTION
Demyelination, i.e. pathological processes that lead to damage to the myelin sheath surrounding axons and ultimately to axonal loss, is a hallmark of multiple sclerosis and various neurodegenerative diseases including Alzheimer’s disease. Novel treatment strategies seek to enhance remyelination and axonal recovery through acceleration of myelin debris clearance by microglia (brain-resident phagocytes). The microglia receptor TREM2 (triggering-receptor expressed on myeloid-cells-2) has been implicated in the regulation of phagocytosis, migration and anti-inflammatory activity1,2. The homozygous TREM2 mutation p.T66M leads to Nasu-Hakola disease which presents with severe demyelination3. Our goal was to further elucidate the role of TREM2 in de- and remyelination. We combined a knock‐in mouse model for the disease‐associated TREM2 p.T66M loss-of-function mutation (T66M-TREM2)4 with feeding of the copper chelator cuprizone for oligodendrocyte depletion as an accepted mouse model for demyelination. De- and remyelination was assessed longitudinally in white and grey matter using clinically translatable multiparametric MRI readouts.METHODS
In three related studies in adult male mice, demyelination was induced with 0.2%cuprizone food-admix for 4-7weeks followed by cuprizone withdrawal to allow for remyelination. Progression and regression of pathology were assessed by multiparametric MRI including T2, magnetisation transfer ratio (MTR), diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) performed prior, during and after cuprizone feeding (see Fig. 1 for detailed study designs).
(A) The cuprizone model as such was characterised with C57Bl/6 mice fed with and without cuprizone.
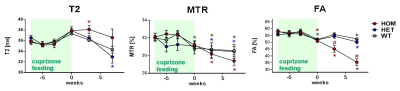
(B) The effects of TREM2 dysfunction was investigated in cuprizone-fed heterozygous (HET), homozygous (HOM) and wild-type (WT) T66M-TREM2 mice.
(C) The former study was replicated in younger T66M-TREM2 animals subjected to shorter cuprizone feeding in order to create a milder and thus more translationally-relevant paradigm and to extend our investigations to grey matter.
MRI was performed on a BioSpec 9.4T/20cm (Bruker BioSpin, Germany) scanner equipped with a body coil and a receive-only brain surface coil. T2 maps were acquired with a multi-slice multi-spin-echo sequence (TR=2200ms, TE=10-120ms, 2averages). MTR were assessed with a RARE-4 sequence (TEeff/TR=16/2365ms, 4averages) preceded by a magnetisation transfer module with 10uT irradiation 8500Hz downfield of water. DTI and DKI were acquired based on the 2-9-9 scheme (2 Ao images and 9 gradient directions at b-values 1000 and 2000, respectively) with an EPI readout (TE/TR=23.5/3000ms, 4averages)6. All images were collected over a 20mmx20mm field-of-view and 15 or 16 slices of 0.6mm and 0.8mm thickness, respectively. For quantification, images were registered to an anatomical template and parameters were calculated over pre-defined brain regions comprising white and grey matter. Tissue and cerebrospinal fluid (CSF) were sampled for analysis of soluble TREM2.
RESULTS and DISCUSSION
Our investigations in C57Bl/6 mice essentially corroborated previous findings in that T2 and MTR as proxies for edema/cellular debris and myelin content, respectively, were significantly altered in corpus callosum after cuprizone feeding and then normalised (partially) in the subsequent wash-out (Fig. 2). Fractional anisotropy determined by DTI as a measure of functional integrity of myelin showed a significant drop that remained throughout the seven weeks of wash-out. This suggests that remyelination occurs but the myelin-sheaths produced differ from the healthy situation and thus may have impaired functionality.
Notably, in cuprizone-fed T66M-TREM2 mice, the HOM animals showed an exacerbated cuprizone effect and further deteriorated during the wash-out period, whereas the difference between HET and WT was minor/inexistent (Fig. 3). These data fully support the pivotal role of TREM2 in fostering and controlling microglia-driven phagocytosis of debris that otherwise hinders remyelination.
In a third study we corroborated these findings in a milder variant with shorter cuprizone feeding in T66M-TREM2 mice (Fig. 4). Assessments of structural integrity were also extended to grey matter by means of DKI. Mean kurtosis as a measure of microstructural complexity increased upon cuprizone feeding and in HOM animals it continued to do so even during the wash-out phase (Fig. 5), which suggests progressive structural disintegration and lack of proper remyelination.
The soluble form of TREM2 in CSF was proposed as a biomarker for microglia activity.5 In our study in C57BL/6 animals, soluble TREM2 was significantly increased for up to 4 weeks after cuprizone removal, thus further witnessing to the importance of TREM2 in the recovery processes from demyelination.
CONCLUSION
Using multiparametric MRI, we demonstrated that TREM2 deficiency leads to progressive structural disintegration and to the absence of proper remyelination in white and grey matter in the cuprizone mouse model. Together with the finding of increased soluble TREM2 during remyelination in wild-type mice, these data underscore the pivotal role of TREM2 in myelin sheath regeneration and provide support for TREM2 as potential treatment target.Acknowledgements
Thanks to Christian Haass and Gernot Kleinberger from Adolf Butenandt Institute, Ludwig-Maximilians-Universität München, Germany for providing the mouse line.References
1. Kleinberger G, Yamanishi Y, Suárez-Calvet M, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014 Jul 2;6(243):243ra86.
2. Mazaheri F, Snaidero N, Kleinberger G, et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 2017 Jul;18(7):1186-1198.
3. Paloneva J, Manninen T, Christman G, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002 Sep;71(3):656-62.
4. Kleinberger G, Brendel M, Mracsko E, et al. The FTD-like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. EMBO J. 2017 Jul 3;36(13):1837-1853.
5. Suárez-Calvet M, Kleinberger G, Araque Caballero MÁ, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer's disease and associate with neuronal injury markers. EMBO Mol Med. 2016 May 2;8(5):466-76.
6. Hansen B, Lund TE, Sangill R, et al. Experimental considerations for fast kurtosis imaging. Magn Res Med. 2016; 78:1455-1468.
Figures