1831
Hippocampus Glutamate Concentrations in Schizophrenia and Bipolar Disorder1Psychiatry, Beth Israel Deaconess Medical Center, Boston, MA, United States, 2Psychiatry, Harvard Medical School, Boston, MA, United States, 3Psychology, University of Georgia, Athens, GA, United States, 4Psychiatry, Yale University, Hartford, CT, United States, 5Psychiatry, University of Chicago, Chicago, IL, United States, 6Psychiatry, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
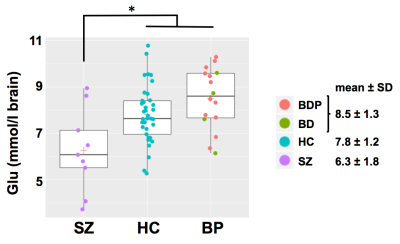
Deficient hippocampus glutamatergic function could underlie cognitive deficits and positive-negative symptoms in schizophrenia (SZ) and bipolar disorder (BP). Using 1H MRS, we found that the glutamate concentration of left anterior hippocampus was significantly lower in SZ (6.3 ± 1.8 mM) vs. healthy controls (HC, 7.8 ± 1.2 mM, p=0.021) and BP (8.5 ± 1.3 mM, p=0.001) and trended higher in BP vs. HC (p=0.179). Decreased glutamate is consistent with deficient excitatory neurotransmission in the hippocampus of patients with SZ, which could alter synaptic plasticity underlying memory and cognition. Our findings are consistent with the glutamate hypothesis of SZ.
Introduction
Alterations of hippocampus excitatory glutamatergic neurotransmission have been implicated in the pathophysiology of schizophrenia (SZ)1,2 and bipolar disorder (BP)3. According to the glutamate (Glu) hypothesis of SZ, deficient glutamatergic function could underlie positive and negative symptoms as well as cognitive deficits. We examined hippocampal concentrations of Glu and other metabolites in SZ, BP and age-matched healthy controls (HC). We hypothesized that Glu would be decreased in SZ.Methods
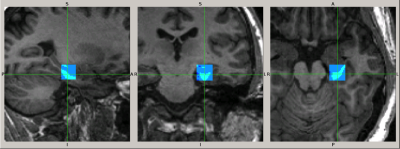
We studied 9 patients with SZ (8 men, 37 ± 12 years), 16 patients with BP (9 men, 34 ± 10 years; 12 with psychotic symptoms: BDP, 4 without: BD) and 37 HC (17 men, 31 ± 10 years). We performed magnetic resonance spectroscopy (MRS) of a single 3.4 ml voxel located in the anterior pole of the left hippocampus (see Fig. 1) using a 3T scanner (GE Signa HDxt), an 8-channel 1H RF head-coil, a PRESS sequence with TE/TR = 35/2000 ms, spectral width = 5000Hz, 4096 complex points and 128 averages. Non-suppressed water signal was acquired with 2 averages for absolute quantitation of metabolites. A high-resolution structural T1-weighted image was acquired for brain tissue segmentation. MRS data were analyzed using LCModel software4 (version 6.3-1L) with water scaling and correction for partial voxel fractions of grey and white matter and cerebrospinal fluid to estimate absolute brain tissue concentrations of Glu, N-acetylaspartate, myo-inositol, total creatine, cholines and Glx (Glu + glutamine). Using R software, we performed between-group comparisons of these metabolite concentrations by ANOVA with gender as a covariate and applied Benjamini-Hochberg multiple comparison correction. Tukey post-hoc analysis was used for pair-wise group comparisons.Results
Hippocampal Glu was significantly lower in SZ (6.3 ± 1.8 mM) compared to HC (7.8 ± 1.2 mM, p = 0.021) and BP (8.5 ± 1.3 mM, p = 0.001); Glu tended to be higher in BP compared to HC (p = 0.179) (see Fig. 2). No other metabolite was significantly different between groups.Conclusions
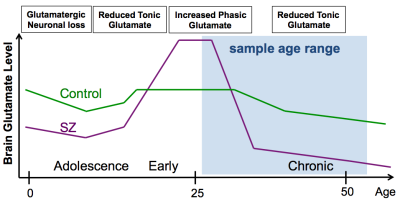
Our finding of significantly decreased Glu is consistent with deficient excitatory glutamatergic neurotransmission in the left anterior hippocampus of patients with SZ. Glutamatergic decrease in this patient group could be explained by a unitary pathophysiological model, in which early course glutamatergic excess leads to excitoxicity followed by glutamatergic failure during the chronic phase of illness5 (see Fig. 3). Glu was slightly higher in BP vs. HC, suggesting distinct glutamatergic alterations in the two patient groups. Hippocampal glutamatergic deficiency could alter synaptic plasticity underlying memory and cognition. The Glu synapse provides several novel pharmacological treatment targets. Future studies will examine the relationship of Glu to cognitive performance and positive and negative symptom scores to determine the potential of these MRS measures as biomarkers to evaluate the efficacy of pharmacological and cognitive behavioral treatments.Acknowledgements
This work was supported by NIMH grant R01 MH78113 (PI: MSK)References
1. Stan, A. D. et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry 20, 433–439 (2015).
2. Merritt, K., Egerton, A., Kempton, M. J., Taylor, M. J. & McGuire, P. K. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry 73, 665–674 (2016).
3. Chitty, K. M., Lagopoulos, J., Hickie, I. B. & Hermens, D. F. Hippocampal glutamatergic/NMDA receptor functioning in bipolar disorder: A study combining mismatch negativity and proton magnetic resonance spectroscopy. Psychiatry Res 233, 88–94 (2015).
4. Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30, 672–679 (1993).
5. Keshavan, M. S. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res 33, 513–521 (1999).
Figures