1830
Trait anxiety associated metabolic alterations in thalamus: An MRS study1NMR Research Centre, Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi, India
Synopsis
Trait anxiety is a prone phenotype for the development of anxiety disorders and depression. Therefore, in order to identify the individuals 'at risk', identifying the hallmarks of trait anxiety becomes important. Ones identified, timely preventive interventions may be given to such individuals. This study is an attempt to study the trait anxiety associated metabolic/ neurochemical alterations in the brain using proton magnetic resonance spectroscopy. We obtained an increase in the concentrations of Choline compounds in the thalamus as a function of trait anxiety of the subjects suggesting an altered cell membrane metabolism.
Introduction
Anxiety disorders are the most prevalent mental disorders affecting up to 33.7% of population during their lifetime (Bandelow and Michaelis, 2015). The disorders are associated with an immense burden on the economy as well as health-care utilization. High anxiety trait, an individual's disposition to experience anxiety related feelings or thoughts, is considered to be a vulnerability factor to develop both depression and anxiety disorders (Spielberger 1983; Sandi C and Richter-Levin G, 2009). Therefore, studying these 'at-risk' subjects and identifying a putative biomarker becomes important to provide timely preventive interventions. In this study we tried to identify potential MR based markers of trait anxiety using invivo proton magnetic resonance spectroscopy (1H MRS). Thalamus is one of the regions that has been implicated in both fear and anxiety circuitry. It integrates sensory input from the primary sensory cortices and sends output to the amygdala (Duval et al., 2015). Thalamus was therefore chosen as the region of interest in the current study. Alterations in neurometabolites in thalamus in relation with trait anxiety levels (assessed by Spielberger’s State-Trait Anxiety Inventory (STAI)(Spielberger 1983)) of the subjects were assessed .Materials and Methods
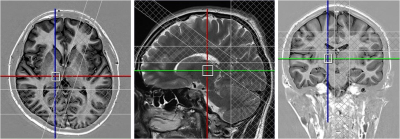
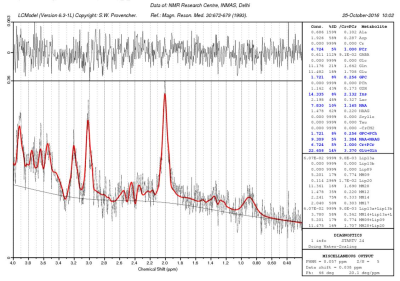
Right-handed healthy educated participants (male – 10, female – 13, mean age+SD= 22.48+2.21 years) participated in the study. The study was carried out using 3T whole body MR system (Magnetom Skyra, Siemens) with a 32 channel head coil. For positioning the MRS voxels, axial and coronal sections were obtained covering the entire brain using Turbo Inversion Recovery Sequence (TR=2900 ms, TE=9.8 ms, TI=500 ms, TA=2 min 10 sec, NEX=1, matrix=270x384, FOV=206x220 mm, 25 slices, slice thickness=2.5 mm, distance factor=0.25 mm). For sagital sections Turbo Spin Echo sequence was used (TR=3600 ms, TE=89 ms, TA=1 min 55 sec, NEX=1, matrix=269 X 384, FOV=180x180 mm, 25 slices, slice thickness=2.5 mm, distance factor=0.25 mm)). A 1.2x1x1.2 cm3 voxel was positioned in the thalamus Figure1. MRS was obtained using PRESS (Point Resolved Spectroscopy) sequence: TR/TE=2000ms/33ms; 2048 spectral points; 1200 Hz spectral Bandwidth and 300 averages with a total acquisition time of 10 min. Automated global shimming and localized shimming were used to minimize the B0 inhomogeneties over the voxel of interest. For spectral quantifications, unsuppressed water (with 10 averages) spectra was also acquired immediately prior to the water suppressed metabolic acquisition. The spectra were processed using LCModel software (Provencher, 1993) (Figure2). The neurochemicals with Cramer Rao Lower Bounds less than 20% were analysed. Concentrations (in institutional units) of myo Inositol, NAA, glycerophosphocholine (GPC), combined peak of glycerophosphocholine and phosphocholinecholine (GPC+PCh), combined peak of NAA and N-acetyl aspartyl glutamate (NAA+NAAG), combined peak of glutamate and glutamine (Glu+Gln) were measured. Participants completed the STAI and Beck Depression Inventory (BDI; Beck et al., 1996) after the scanning session. A partial correlation analysis was carried out between the neurometabolite concentrations and the trait anxiety scores of the subjects. Age, sex, BDI scores, Full Width at Half maxima (FWHM) and Signal to Noise ratio (SNR) of the spectra were taken as covariates of no interest.Results
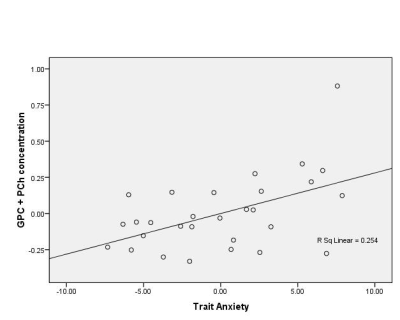
The descriptive statistics for self report measures are as follows: STAI trait anxiety scores = 34.26 + 4.82 (range: 28-42), BDI scores = 5.22 + 2.76 (range: 0-11). Partial correlation analysis revealed a positive correlation between the concentrations of both GPC (r =0.540, p = 0.021) and GPC+PCh (r =0.540, p = 0.021) (Figure3) in the thalamus with the trait anxiety scores of the subjects.Discussion
Choline plays a vital role in cell membrane metabolism and has an important role in the central nervous system for the synthesis of membrane phosphatidylcholine and acetylcholine. Choline levels have been linked to the neuropathology of mood disorders in earlier 1H-MRS studies. Choline has been found to be increased in depressed subjects in adult and pediatric samples (Vythilingam et al., 2003). An increase in choline was reported in thalamus, parietal white matter and hippocampus of Obsessive Compressive Disorder patients (Brennan et al., 2013) suggesting an increased membrane turnover from membrane breakdown or synthesis. The changes in the choline resonance may be reflective of changes in local neuronal metabolism that may be required for the incorporation of choline compounds into phospholipids (MacMaster and Kusumakar, 2006). Increased Choline was also reported in the orbito frontal cortex of healthy subjects with high trait anxiety as compared to their low trait anxiety counterparts (Grachev ID and Apkarian, 2000). Our study provides preliminary evidence to Choline alterations in the thalamus of healthy individuals as a function of their trait anxiety levels thereby suggesting a role of choline in mediating the physiological and behavioural consequences associated with anxiety at sub-clinical level.Acknowledgements
The work was supported by R&D project No. INM 311 of the Defence R&D Organisation, Ministry of Defence, India.References
1. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience. 2015;17(3):327-335.
2. Spielberger CD. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. 1983.
3. Sandi C and Richter-Levin G. From high anxiety trait to depression: a neurocognitive hypothesis. Trends in Neuroscience. 2009; 32 (6): 312-320.
4. Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management. 2015;11:115-126.
5. Provencher, S.W. Estimation of metabolite concentrations from localized in vivo NMR spectra. Magnetic Resonanace in Medicine. 1993; 30: 672–679.
6. Beck AT, Steer, R.A., Brown, G.K. Beck depression inventory: manual. 2nd ed. San Antonio: The Psychological Corporation. 1996.
7. Vythilingam M, Charles HC, Tupler LA, Blitchington T, Kelly L, Krishnan KR. Focal and lateralized subcortical abnormalities in unipolar major depressive disorder: an automated multivoxel proton magnetic resonance spectroscopy study. Biol Psychiatry. 2003; 54(7): 744-50.
8. Brennan BP, Rauch SL, Jensen JE, and Pope Jr HG. A critical review of magnetic resonance spectroscopy studies of Obsessive Compulsive Disorder. Biol Psychiatry. 2013; 73(1): 24–31.
9. MacMaster FP and Kusumakar V. Choline in Pediatric Depression McGill Journal of Medicine : MJM. 2006; 9: 24-27.
10. Grachev ID and Apkarian AV. Chemical Mapping of Anxiety in the Brain of Healthy Humans: An in vivo 1H-MRS Study on the Effects of Sex, Age, and Brain Region. Human Brain Mapping. 2000; 11: 261–272.
Figures