1828
A pilot study of cerebral blood flow changes in patients undergoing electroconvulsive therapy1Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 2Psychiatry, Stony Brook Medicine, Stony Brook, NY, United States, 3Radiology, Stony Brook Medicine, Stony Brook, NY, United States
Synopsis
Electroconvulsive therapy (ECT) is an effective choice for patients with untreatable depression. Although it is very effective, the mechanisms through which ECT works are poorly understood. We have previously collected PET/MRI data in patients receiving ECT which suggest that this treatment strongly affects the hippocampus. Herein, we supplement these preexisting data with arterial spin labeling data showing significantly reduced blood flow to the hippocampus following ECT in three responders.
Introduction
Electroconvulsive therapy (ECT) remains the most efficacious treatment for patients with treatment-resistant depression. Despite over 75 years of documented successful use, the mechanisms by which ECT exerts its therapeutic benefits remain poorly understood. We have previously reported neuroimaging evidence of increased hippocampal neurogenesis following successful ECT. Here, we provide a further analysis of cerebral blood flow (CBF) in the hippocampi of a subset of the previously reported population1.Methods
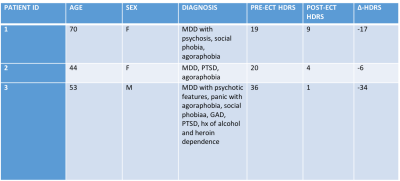
Arterial spin labeling (ASL) data were acquired in 3 patients (age: 44, 53, 70) undergoing clinically indicated ECT due to treatment-resistant depression. PET/MRI data were acquired before and after treatment, as were 17-item Hamilton Depression Rating Scale (HDRS) scores. All images were acquired on a 3T Siemens Biograph mMR.
ECT was administered as per the recommendations set forth by the American Psychiatric Association Task Force on ECT. Patients received three treatments per week, the total number of treatments administered was determined individually for each subject by their treating physician (AY). Patients were treated bilaterally using a Thymatron System IV.
Cerebral blood flow (CBF) was mapped using pseudo-continuous ASL with background suppression and segmented 3D-GRASE acquisition (TR/TE/Label Time/Post Labeling Delay=4000/20/1600/1500 ms). Background suppression pulse timings were optimized for ~90% reduction of static tissue signal. An additional reference image was acquired with TR of 7000 ms. In-house customized software built upon ASLtbx2 and SPM123 were used for processing ASL data and partial volume correction of CBF maps. The hippocampus was segmented from MPRAGE (TR/TE/TI=2300/2.98/900 ms) using Freesurfer v5.34.
Baseline and post-treatment CBF were assessed for statistically significant differences using a paired t-test. P-values of less than 0.05 are taken to be significant. Analysis was performed using MATLAB 2016b.
Results
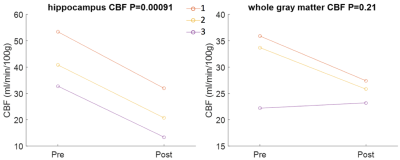
All three patients were ECT responders, and two were remitters. CBF to the bilateral hippocampi (p=0.00091) was seen to decrease following ECT. We observed significant reductions in both the left (p=0.0143) and right (p=0.0128) hippocampus when analyzing them independently. Whole-brain gray matter CBF was seen to decrease in two of these individuals; a small increase was observed in the patient with considerably lower baseline CBF. The whole brain gray matter reduction did not attain statistical significance (p=0.21). Pre- and post-treatment CBF is shown in Figure 1.Discussion
This work is novel, as no studies to date have assessed hippocampal CBF in relation to ECT. We observed significant reductions in hippocampal CBF in three patients who responded to ECT. Moreover, we observed a reduction in whole brain gray matter CBF in two of these three responders, although the observation failed to reach statistical significance. Previously studies using SPECT have shown that whole brain CBF is reduced following ECT5,6, suggesting that individuals who respond to ECT achieve CBF values similar to healthy controls7. These data, taken with our primary observation of significantly reduced CBF to the hippocampi, further our previously stated hypothesis that successful ECT normalizes hippocampal function and, in particular, neurogenesis, in individuals who respond to this treatment.
It is worthy to note that this analysis is limited by the number of subjects analyzed. ASL data in the remaining1, previously analyzed and reported, patients were rejected prior to analysis due to motion artifacts.
Conclusion
Reductions in hippocampal CBF further the theory put forth in our previous analysis that successful ECT normalizes hippocampal functioning.Acknowledgements
No acknowledgement found.References
1. Huang C, Kunkel L, Yacoub A, Ding J, DeLorenzo C, Parsey R. Changes in Amygdala Connectivity and Hippocampal Volumes after Electroconvulsive Therapy: A Pilot MRI Study. In. ISMRM Annual Meeting 2016.
2. Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic resonance imaging. 2008;26(2):261-269.
3. Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical parametric mapping: the analysis of functional brain images. Academic press; 2011.
4. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355.
5. Rosenberg R, Vorstrup S, Andersen A, Bolwig TG. Effect of ECT on cerebral blood flow in melancholia assessed with SPECT. The Journal of ECT. 1988;4(1):62-73.
6. Nobler MS, Sackeim HA, Prohovnik I, et al. Regional cerebral blood flow in mood disorders, III: treatment and clinical response. Archives of general psychiatry. 1994;51(11):884-897.
7. Mann JJ, Kupfer DJ. Biology of depressive disorders. Part A: A systems perspective. Vol 3: Springer Science & Business Media; 2013.