1826
Increased functional connectivity between medial prefrontal cortex and nucleus accumbens in morphine craving rats1CMRR, Department of Radiology, University of Minnesota Medical School, Minneapolis, MN, United States, 2MnDRIVE Optogenetics and Neuromodulation Core, Neuroscience Department, University of Minnesota Medical School, Minneapolis, MN, United States, 3Departments of Neuroscience and Psychology, University of Minnesota Medical School, Minneapolis, MN, United States
Synopsis
Morphine is a potent analgesic with a high addictive potential. In this study we have shown a difference in brain connectivity related to drug-seeking behavior involving key neural decision and reward systems using rs-fMRI. The finding contributes to a better understanding of the neural underpinnings of opioid addiction and could help in a better assessment of relapse risk in individuals.
Introduction
The current opioid addiction crisis is an urgent mental health problem, requiring a better understanding of underlying neural mechanisms to design more effective brain-based treatments. Non-invasive fMRI approaches have the promise to define the changes in neural reward circuits produced during different phases of addiction-like behavior in rodent models of addiction1–3. It is known that the prefrontal input to the nucleus accumbens (NAc), a key area of reward processing, can become significantly disrupted after multiple administrations of morphine. Specifically, the glutamatergic input from infralimbic (IL) and prelimbic (PrL) areas undergoes AMPA-receptor specific plasticity, resulting in synaptic strengthening in the NAc as shown by electrophysiology. In this resting-state fMRI (rs-fMRI) study, we aimed to detect this influence through changes in resting state connectivity between those involved brain areas under the assumption that a stronger connectivity results in a higher temporal correlation of resting state low frequency BOLD fluctuations between the NAc and the frontal areas. This rs-fMRI study was conducted at high field of 9.4T for improving BOLD sensitivity and spatial specificity enabling the functional mapping of the connectivity change in a model of morphine craving.Materials and Methods
Six total Sprague-Dawley rats (5 female, 1 male; 250-300g BW) were used in this study (3 female rats were administered morphine (10 mg/kg, i.p.), according to a previously established protocol to induce sensitization3). Isoflurane anesthetized animals under orotracheal ventilation at varied anesthetic depth (1.2-1.5%) were maintained at ~37°±0.7C by a water bath. Procedures were approved by the IACUC Committee of the University of Minnesota.
The MRI study was performed on a 9.4T animal scanner (Varian Inc.) using a custom-built single loop proton RF surface coil. GE-EPI images (TE = 16.5ms) were acquired at temporal resolution of 1s (except one dataset at TR=0.65s), 500 volumes at in-plane 0.5x0.5mm2 voxel size and 0.75mm slice thickness. Data were analyzed both individually and on a group level5 using the seed (in NAc with a surrounding +0.5mm) based correlation methods in AFNI using InstaCorr. Pre-processing included motion correction, temporal bandpass filtering between 0.01-0.2Hz, Gaussian smoothing (FWHM=0.5mm). Functional and anatomical MRI data were coregistered to a Waxholm Atlas4 and seeds were placed in each left and right nucleus accumbens to perform correlation analysis of resting-state connectivity5.
Results
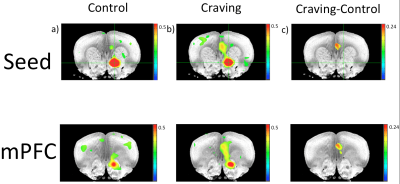
The connectivity between the NAc and mPFC area over a cluster extending anterior-posterior along the IL/PrL system was found significantly strengthened in the morphine sensitized (“craving”) rats with a higher CC value (Fig. 1b) as compared to the control group (Fig. 1a). Figure 1c is the difference map of the connectivity between craving and control based on the group data analysis. Only in the right NAc seed a sufficient differentiation of connectivity within mPFC was observed, although a tendency of enhancing the striatal to frontal connectivity was observed in all craving rats, and the tendency became weak when anesthetic depth was deepened.Discussion
In healthy controls, the resting-state connectivity between NAc and mPFC is relatively weak as compared to other much stronger networks (e.g. somatosensory). Therefore, it is not too surprising that only in the craving condition, which supposedly increases the connection strength, a statistically significant correlation can be observed. Our finding about strong connection between NAc and mPFC is in agreement with electrophysiological studies3 as well as task-based fMRI of acute drug consumption in rodents1. Through this non-invasive rs-fMRI assessment, we can augment and cross-check behavioral changes usually indicative of drug-seeking subjects3.
Moreover, the fact that differences in brain connectivity were found at low Isoflurane concentrations highlights the importance of conducting further studies in light anesthesia or awake trained animals for improving BOLD contrast-to-noise ratio and mapping specificity6.
Conclusion
We have shown a difference in brain connectivity related to drug-seeking behavior involving key neural decision and reward systems using rs-fMRI. The finding contributes to a better understanding of the neural underpinnings of opioid addiction and could help in a better assessment of relapse risk in individuals.Acknowledgements
NIH Grants: R01 MH111447, R24 MH106049, P41 EB015894, P30 NS076408; WM Keck Foundation and University of Minnesota Wallin Family Foundation.References
1. Febo, M. et al. The Neural Consequences of Repeated Cocaine Exposure Revealed by Functional MRI in Awake Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 30, 936–943 (2005).
2. Liang, Z., Li, T., King, J. & Zhang, N. Mapping thalamocortical networks in rat brain using resting-state functional connectivity. NeuroImage 83, 237–244 (2013).
3. Hearing, M. C. et al. Reversal of morphine-induced cell-type–specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc. Natl. Acad. Sci. 113, 757–762 (2016).
4. Papp, E. A., Leergaard, T. B., Calabrese, E., Johnson, G. A. & Bjaalie, J. G. Waxholm Space atlas of the Sprague Dawley rat brain. NeuroImage 97, 374–386 (2014).
5. Chen, G., Saad, Z. S., Nath, A. R., Beauchamp, M. S. & Cox, R. W. FMRI group analysis combining effect estimates and their variances. NeuroImage 60, 747–765 (2012).
6. Zhang, N. et al. Mapping resting-state brain networks in conscious animals. J. Neurosci. Methods 189, 186–196 (2010).
Figures