Kangkang Xue1, Dandan Zheng2, and Jingliang Cheng1
1Medical Imaging and Nuclear Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2GE Healthcare, China, Beijing, China
Synopsis
Schizophrenia is a chronic mental illness whose symptoms are
thought to have a strong neurobiological basis. This work is to study the
resting state networks changes in first-episode schizophrenia patients by
resting-state functional magnetic resonance imaging. The current study explored
that there were RSNs damages or multiple brain regions functional connectivity
abnormalities in first-episode schizophrenia patients compared with healthy
controls, which behave functional connectivity increase and decrease.
Introduction
Schizophrenia is
a chronic mental illness whose symptoms are thought to have a strong
neurobiological basis. This work is to study the resting state networks (RSNs)
changes in first-episode schizophrenia patients by resting-state functional magnetic
resonance imaging (fMRI).Methods
Forty-eight
patients with first-episode schizophrenia and forty healthy age-matched controls
were recruited at the First Affiliated Hospital of Zhengzhou University. Eight of
all the subjects were excluded due to the exceeded head movement, therefore, 45
first-episode schizophrenia patients and 35 healthy controls were scanned by a
3.0 Tesla MR scanner (Discovery MR750, General Electric, Milwaukee, WI, USA). Functional
MRI data were obtained using a single-shot GRE-EPI sequence (TR/TE = 2000/41
ms; field of view = 220×220mm
2; matrix = 64×64; flip angle = 90°;
slice thickness = 3 mm; 1mm gap; 34 slices; 190 time points). Anatomical images
were acquired using a T1-weighted 3D SPGR sequence (TR/TE = 8.2/3.2 ms; FOV =
256×256 mm
2; matrix = 256×256; slice thickness = 1.0 mm, no gap; 188
slices).
Resting-state
fMRI data pre-processing was conducted with the DPARSFA software
package. Pre-processing steps include: format conversing, excluding time point,
time correction, head movement correction, spatial normalization and spatial
smoothing. After then, independent component analysis(ICA) were performed on
fMRI data to identify RSNs associated with schizophrenia and healthy controls using
GIFT software. Differentiations of functional connectivity in each RSN between
schizophrenia and control groups were computed and analyzed using SPM8 software.
The voxel-wise two sample t-test (FDR-corrected) were performed to compare the
group differences in function connectivity of each RSN.
Results
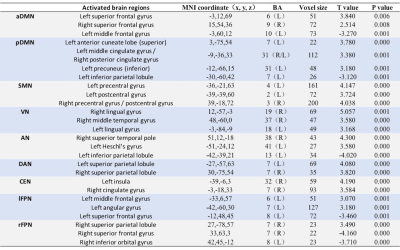
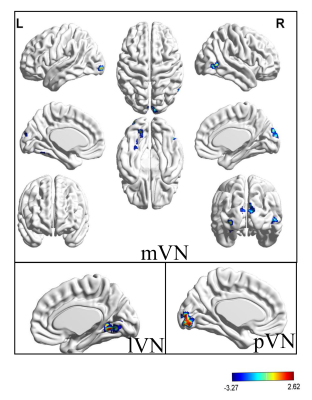
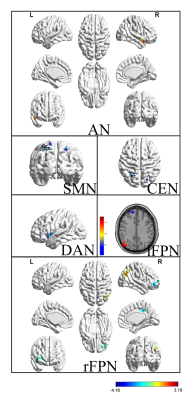
Eleven RSNs, including
anterior default network front (aDMN), posterior default network (pDMN),
sensorimotor network (SMN), medial visual network (mVN), lateral visual network
(lVN), occipital pole visual networks (pVN), auditory network (AN), dorsal
attention network (DAN), left frontoparietal network (lFPN), right
frontoparietal network (rFPN) and central-executive network (CEN), derived from
first-episode schizophrenia and healthy controls were obtained. Compared with
healthy controls, first-episode schizophrenia showed significantly increased or
decreased function connectivity (p<0.05, FDR-corrected, voxel level) in some
brain regions of each RSN as indicated in Table 1. In detail, positive T value
means first-episode schizophrenia had significantly increased function
connectivity in some brain region than that of healthy control groups, while
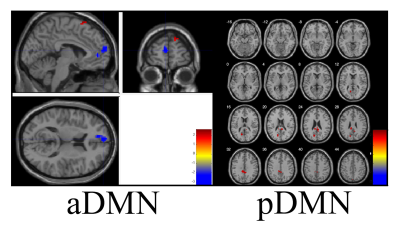
negative T value is the opposite. Take aDMN for example, compared with healthy
control groups, three abnormal brain regions including left frontal gyrus
(t=3.840, p<0.05), right frontal gyrus (t=2.514, p<0.05) and left medial
frontal gyrus (t=-3.270, p<0.05) were found in this study which showed
significantly change in function connectivity. At the same time, corresponding
T value maps of eleven RSNs were showed in Figure 1-3.
Discussion
Previous studies
have shown that the default network is perturbed in people suffering from
schizophrenia
[1, 2]. The post cingulate cortex (PCC), precuneus, medial
prefrontal cortex (MPFC) and anterior cingulate cortex (ACC) of DMN are the
main brain region for higher cognition function, which is involved in the
processes of higher cognition and many psychiatric disorders
[3]. In this
study, the finding of abnormal functional connectivity in the frontal lobe,
precuneus and cingulate is consistent with previous study above which is
related to episodic memory and high cognition dysfunction.
Central-executive
network in human brain is related to signal processing which guide human
response to any stimulation. The finding of increased function connectivity in
left insular lobe and right cingulate of first-episode schizophrenia reveals
that there is feedback loop damage in patients’ brain.
Previous studies
have demonstrated that there are not only some abnormity of function
connectivity and lateralization index in FPN, but also high correlation with
score of schizophrenia
[4]. In this study, abnormity of function connectivity
in some brain regions of bilateral frontal parietal network also has been
found, and there may be related to some impairment of language, memory and
attention in first-episode schizophrenia patients.
Salience network
including the insula lobe and the anterior cingulate is an important network in
schizophrenia research. Numerous resting-state fMRI studies
[5-9] have revealed high correlation
between the abnormity of this network and symptoms of schizophrenia, such as delusion
and photism. In this study, the main overlapping brain regions of salience
network showed significantly change between two groups.
Conclusion
In conclusion, function
connectivity from different networks may help explain the complex relationships
between distributed cerebral sites in the brain and possibly provide new
understanding of neurological and psychiatric disorders such as schizophrenia. The current study explored that there
were RSNs damages or multiple brain regions functional connectivity
abnormalities in first-episode schizophrenia patients compared with healthy
controls, which behave functional connectivity increase and decrease.
Acknowledgements
No acknowledgement found.References
[1] Raichle M E, et al. Proceedings of the
National Academy of Sciences of the United
States of
America, 2001.
[2] Gusnard D, et al. Nature Reiview
Neuroscience, 2001.
[3] Simone, et al. Schizophrenia Bulletin, 2013.
[4] Anna R J, et al. Schizophrenia Research,
2010.
[5] White T P, et al. Schizophrenia Research,
2010.
[6] Menon V, et al. Brain Structure &
Function, 2010.
[7] Palaniyappan L, et al. Journal of Psychiatry
& Neuroscience Jpn, 2012.
[8] Wang Y, et al. Chinese Journal of Nervous
& Mental Diseases, 2013.
[9] Orliac F, et al. Schizophrenia Research,
2013.