1822
Myelin content and axonal size/density is reduced in early-course schizophrenia: Evidence from multi-echo T2 imaging study1MD Program, Wayne State University School of Medicine, Detroit, MI, United States, 2Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States, 3MD/PhD Program, Wayne State University School of Medicine, Detroit, MI, United States, 4Psychology, Wayne State University, Detroit, MI, United States, 5Institute of Gerontology, Wayne State University, Detroit, MI, United States
Synopsis
White matter aberrations have been well documented in schizophrenia using diffusion tensor or weighted imaging, but the differences in myelin macrostructure morphology have not been extensively explored. Here we used multi-echo T2 (ME-T2) imaging to examine myelin content and axonal size and packing density in schizophrenia in white matter regions, specifically association, commissural, and projection fiber tracts. We demonstrate reduced myelin content as well as increased axonal packing density in association and projection tracts, which may contribute to neural dysconnectivity mechanisms underlying the neuropathology of schizophrenia.
Introduction
Schizophrenia is the consummated “dysconnection” syndrome1, yet direct anatomical evidence from the perspective of the myelin macrostructure in white matter is lacking2,3,4,5. The myelin sheath that wraps around axons or white matter fibers plays a key role in transmitting signal across fibers. The speed at which signals are transmitted is directly proportional to the number of wraps of the myelin sheath around axons, which also correlates with caliber of axons. A disruption in the myelination of axons, either in myelin wraps (myelin content) or axonal size, may, therefore, cause a dysfunction in the transmission of signal in white matter fiber and should be a proximate anatomical correlate for dysconnection in the illness. Recent advances demonstrate that quantitative measurements reflecting myelin content and axonal size/density can be estimated by modeling the multi-T2 components of water in ME-T2 imaging data6,7,8. The signal amplitude of the short T2 component reflecting water trapped in between myelin layers is expressed as a myelin water fraction (MWF) relative to the total water signal. The intermediate T2 relaxation time constant of the larger intracellular/extracellular water compartment (termed geometric mean or geomT2IEW) reflects axon size and axon density8,9. Previous ME-T2 studies have shown a reduction in MWF of individuals with schizophrenia10,11, but did not target quantitation of myelin content and axonal size packing of key white matter tracts. Here we conduct the first known analyses of these metrics in young early-course schizophrenia patients (<5 years from illness onset).Methods
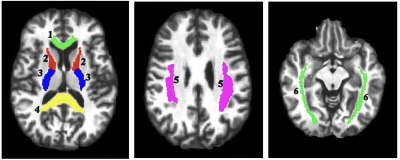
A total of 17 DSM-V diagnosed early-course schizophrenia patients (mean age 26.2±3.5yrs old; 10 males and 7 females) and 25 healthy individuals (mean age 25.5±3.5yrs old; 15 males and 10 females) provided informed consent to participate in the ME-T2 acquisition. Patients were stabilized on antipsychotic medication for at least 3 months and were within 5 years of the their first hospitalization. ME-T2 was acquired on a 3T Siemens Verio system using either a 12 or 32-channel receive-only head coil and a 3D-GRASE sequence (32 echoes, 11 ms inter-echo spacing, TR=1,110 ms, FOV= 222x165 mm2, matrix size= 192x144 in the axial plane and 24 5-mm thick slices). Six regions of interest (ROI) in key white matter areas were examined including projection fibers [anterior and posterior limb of the internal capsule (ALIC and PLIC)], commissural fibers [genu and splenium of the corpus callosum] and association fibers [superior longitudinal fasciculus (SLF), and the inferior fronto-occipital fasciculus (IFOF)]. The ROIs were identified using the JHU White Matter Atlas in standard space, projected onto the subjects’ space, and used to extract the ME-T2 data (Figure 1). Regularized NNLS was used to fit the ME-T2 data with 200 logarithmically spaced T2 values from 10-2,000 ms. The geomT2IEW component was defined as T2 values between 40 and 200 ms. A repeated measures GEE approach with side (right and left), age and sex as covariates was fit to the MWF and geomT2IEW data for each ROI.Results
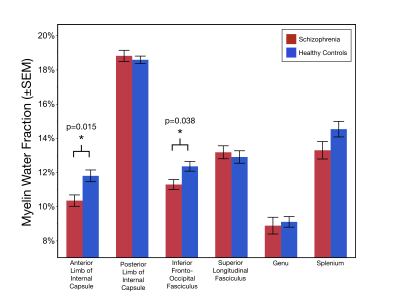
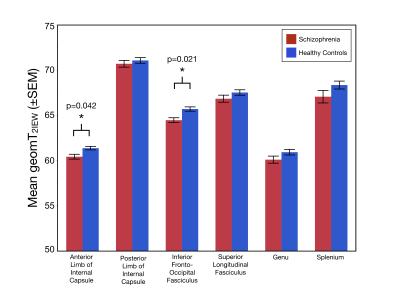
Age and the sex distribution did not differ between the two groups. As shown in Figure 2, MWF values were significantly lower in the ALIC (χ2=5.94;p=0.015) and the IFOF (χ2=4.29;p=0.038) of schizophrenia patients compared to healthy controls. Additionally, geomT2IEW values were significantly lower in the ALIC (χ2=4.15;p=0.042) and in the IFOF (χ2=5.32;p=0.021) of schizophrenia patients compared to healthy controls (Figure 3).Conclusions
Quantification of the multi-T2 water components in representative white matter tracts (association, commissural, and projection fibers) provides the first evidence of both reduced myelin content and smaller axonal size (increased packing density) in two specific white matter tracts, the ALIC and IFOF, in early-course schizophrenia patients. The dysconnection hypothesis posits that anatomical and neurodevelopmental alterations in schizophrenia are consequences of abnormal neuromodulation1. Given that in healthy adults, both MWF and geomT2IEW increase with age, reflecting continued axonal myelination and axonal enlargement well into middle adulthood, the reduction in MWF and geomT2IEW in the ALIC and IFOF in early-course schizophrenia patients suggests an altered progression in the myelination of axons and myelin distribution. As the ALIC and IFOF tracts are involved in sensory and cortical feedback networks, further investigation is ongoing to associate these myelin-related alterations with symptomatology and cognitive deficits in support of the dysconnection hypothesis in schizophrenia.Acknowledgements
This study was supported by NIMH under award number R01 MH111177 (JAS and VAD) and NIA under award number R37 AG011230 (NR), as well as by the Lycaki-Young Funds from the State of Michigan.References
1. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophrenia Research. 2016;176(2): 83–94.
2. Cookey J, Bernier D, Tibbo PG. White matter changes in early phase schizophrenia and cannabis use: an update and systematic review of diffusion tensor imaging studies. Schizophrenia Research. 2014;156 (2):137–142.
3. Iwatani J, Ishida T, Donishi T, Ukai S, Shinosaki K, Terada M, Kaneoke Y. Use of T1-weighted/T2-weighted magnetic resonance ratio images to elucidate changes in the schizophrenic brain. Brain and Behavior. 2015;5(10): e00399.
4. Mendelsohn A, Strous RD, Bleich M, et al. Regional axonal abnormalities in first episode schizophrenia: Preliminary evidence based on high b-value diffusion weighted imaging. Psychiatry Research: Neuroimaging. 2006; 146(3):223-229.
5. Fei Du, Cooper AK, Thida T, et al. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biological Psychiatry. 2013; 74(6):451-457.
6. MacKay A, Whittall K, Mädler J, et al. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31(6):673–677.
7. Arshad, M, Stanley, JA, Raz N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. NeuroImage. 2016;143:26-39.
8. Arshad M. Stanley JA, Raz N. Test-retest reliability and concurrent validity of in vivo myelin content indices: Myelin water fraction and calibrated T1w/T2w image ratio. Human Brain Mapping. 2017; 38(4):1780-1790.
9. Dula AN, Gochberg DF, Valentine HL, Valentine WM, Does MD. Multiexponential T2, magnetization transfer, and quantitative histology in white matter tracts of rat spinal cord. Magnetic Resonance in Medicine. 2010;63 (4):902–909.
10. Flynn SW, Lang DJ, MacKay AL, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Molecular Psychiatry. 2003;8(9): 811–820.
11. Lang DJM, Yip E, MacKay A, et al. 48 echo T2 myelin imaging of white matter in first-episode schizophrenia: Evidence for aberrant myelination. NeuroImage Clinical. 2014;6: 408-414.
Figures